Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0JUOLN
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
MDX-1203
|
|||||
| Synonyms |
BMS 936561; BMS-936561; BMS-936561, MDX-1203; BMS936561; MDX 1203; MDX-1203; MDX1203
Click to Show/Hide
|
|||||
| Organization |
Medarex, Inc.; Bristol Myers Squibb Co.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
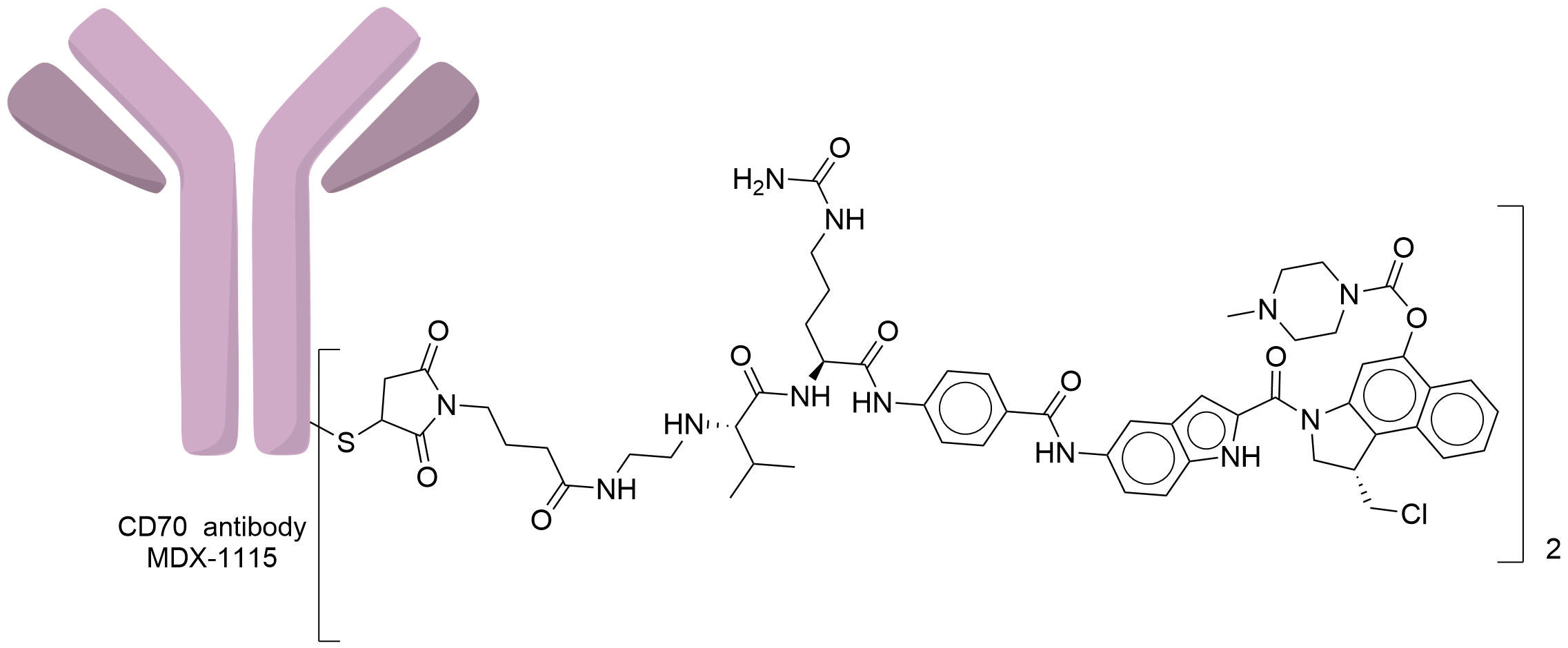
|
|||||
| Antibody Name |
MDX-1115
|
Antibody Info | ||||
| Antigen Name |
CD70 antigen (CD70)
|
Antigen Info | ||||
| Payload Name |
Seco-MED-A
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-Val-Cit
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Puchem SID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
Advanced or recurrent clear cell renal cell carcinoma (ccRCC) or relapsed or refractory B-non Hodgkin lymphoma (NHL), life expectancy of 12 weeks; ECOG performance status of 0-2; measurable disease by RECIST 1.1 criteria for ccRCC and by IWG 2007 criteria for B-NHL as well as adequate hematologic, renal and liver function parameters.
|
||||
| Administration Dosage |
0.50, 1.00, 2.00, 4.00, 8.00, 15.00 mg/kg administered every 21 days in a 42 day cycle for a maximum of 17 cycles, IV.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00944905 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, multicenter, open-label, dose-escalation, multidose study of MDX-1203 in subjects with advanced/recurrent clear cell renal cell carcinoma or relapsed/refractory B-cell non Hodgkin's lymphoma. | ||||
| Primary Endpoint |
The highest best tolerated dose and the recommended dose for future studies was 8 mg/kg dose.There was no MTD determined during the acute toxicity assessment window according to protocol-defined DLT according to protocol-defined DLT. There was disease stabilization in 18 of 26 patients (69.23%) without correlation with received dose.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00944905 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, multicenter, open-label, dose-escalation, multidose study of MDX-1203 in subjects with advanced/recurrent clear cell renal cell carcinoma or relapsed/refractory B-cell non Hodgkin's lymphoma. | ||||
References
