Linker Information
General Information of This Linker
| Linker ID |
LIN0YXWRQ
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Mal-Val-Cit
|
|||||
| Linker Type |
Cathepsin-cleavable linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
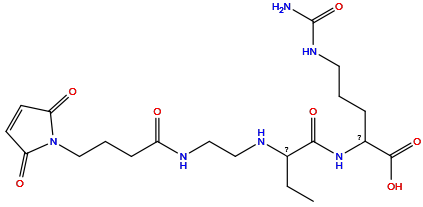
|
|||||
| Formula |
C20H32N6O7
|
|||||
| Isosmiles |
CCC(NCCNC(=O)CCCN1C(=O)C=CC1=O)C(=O)NC(CCCNC(N)=O)C(=O)O
|
|||||
| InChI |
InChI=1S/C20H32N6O7/c1-2-13(18(30)25-14(19(31)32)5-3-9-24-20(21)33)22-10-11-23-15(27)6-4-12-26-16(28)7-8-17(26)29/h7-8,13-14,22H,2-6,9-12H2,1H3,(H,23,27)(H,25,30)(H,31,32)(H3,21,24,33)
|
|||||
| InChIKey |
VOSBZJYYHPWAPI-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
468.511
|
Polar area
|
200.03
|
||
|
Complexity
|
468.2332474
|
xlogp Value
|
-1.8061
|
|||
|
Heavy Count
|
33
|
Rot Bonds
|
16
|
|||
|
Hbond acc
|
7
|
Hbond Donor
|
6
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
MDX-1203 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
Advanced or recurrent clear cell renal cell carcinoma (ccRCC) or relapsed or refractory B-non Hodgkin lymphoma (NHL), life expectancy of 12 weeks; ECOG performance status of 0-2; measurable disease by RECIST 1.1 criteria for ccRCC and by IWG 2007 criteria for B-NHL as well as adequate hematologic, renal and liver function parameters.
|
||||
| Administration Dosage |
0.50, 1.00, 2.00, 4.00, 8.00, 15.00 mg/kg administered every 21 days in a 42 day cycle for a maximum of 17 cycles, IV.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00944905 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, multicenter, open-label, dose-escalation, multidose study of MDX-1203 in subjects with advanced/recurrent clear cell renal cell carcinoma or relapsed/refractory B-cell non Hodgkin's lymphoma.
|
||||
| Primary Endpoint |
The highest best tolerated dose and the recommended dose for future studies was 8 mg/kg dose.There was no MTD determined during the acute toxicity assessment window according to protocol-defined DLT according to protocol-defined DLT. There was disease stabilization in 18 of 26 patients (69.23%) without correlation with received dose.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00944905 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, multicenter, open-label, dose-escalation, multidose study of MDX-1203 in subjects with advanced/recurrent clear cell renal cell carcinoma or relapsed/refractory B-cell non Hodgkin's lymphoma.
|
||||
References
