Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0IBTWL
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
DAR2-ARC-ADC
|
|||||
| Synonyms |
Anti-hCLL-1 ARC-ADC DAR2
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
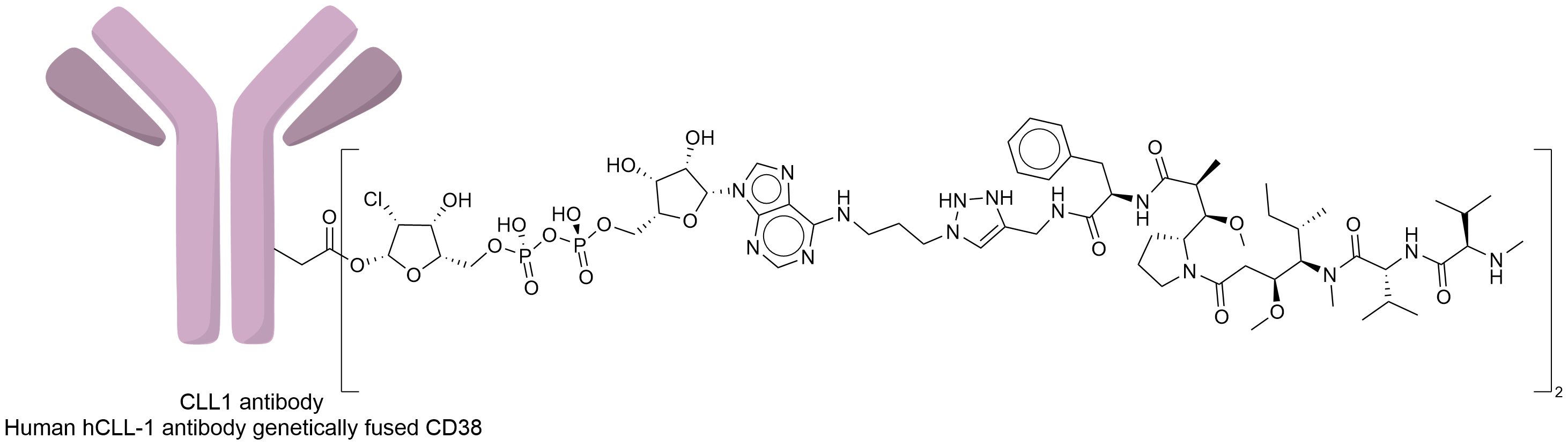
|
|||||
| Antibody Name |
Human hCLL-1 Anti-ody genetically fused with CD38
|
Antibody Info | ||||
| Antigen Name |
Collectin-10 (COLEC10)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin F
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
NAD-based dinucleotide linker
|
Linker Info | ||||
| Conjugate Type |
MMAF was covalently attached to antibodies genetically fused with CD38 via ADP-ribosyl cyclase catalytic glutamate 226 (Glu226) residue, conjugating through a 2-Cl-araNAD group.
|
|||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.90 nM | Positive CLL-1 expression (CLL-1+++/++) | ||
| Method Description |
In vitro cytotoxicity of the anti-hCLL-1 ARC-ADCs was then evaluated using human AML cell lines U937 (CLL-1+) and KG1a (CLL-1-1).
|
||||
| In Vitro Model | Adult acute monocytic leukemia | U-937 cells | CVCL_0007 | ||
References
