Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0FQFDY
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Trastuzumab-SG3227
|
|||||
| Synonyms |
Her-SG3227
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2.22
|
|||||
| Structure |
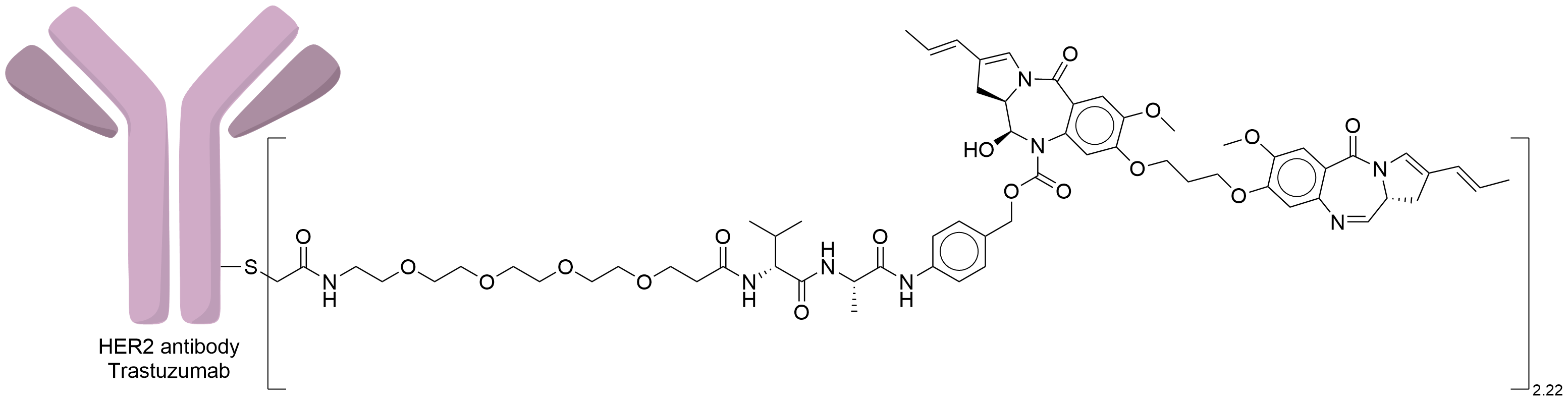
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
SG2219
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Acetamide-PEG4-Val-Ala-PABA
|
Linker Info | ||||
| Conjugate Type |
Partial reduction of the trastuzumab inter-chain disulfide bonds to release cysteine thiols, then react with the iodoacetamide-containing payload.
|
|||||
| Combination Type |
SG3227
|
|||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 1.48 ng/mL | Low HER2 expression (HER2+) | ||
| Method Description |
The cytotoxic effect of Trastuzumab-SG3227 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing breast and gastric cancers. The potency of Trastuzumab-SG3227 was assessed on the NCI-N87 cell line.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 2130.00 ng/mL | Negative expression (HER2-) | ||
| Method Description |
The cytotoxic effect of Trastuzumab-SG3227 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing breast and gastric cancers. The potency of Trastuzumab-SG3227 was assessed on the MDA-MB-468 cell line.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
References
