Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0FMCLF
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
JSKN-003
|
|||||
| Synonyms |
JSKN 003; JSKN-003; JSKN003
Click to Show/Hide
|
|||||
| Organization |
Alphamab Oncology; Alphamab Australia Co. Pty Ltd.
|
|||||
| Drug Status |
Phase 3
|
|||||
| Indication |
In total 5 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
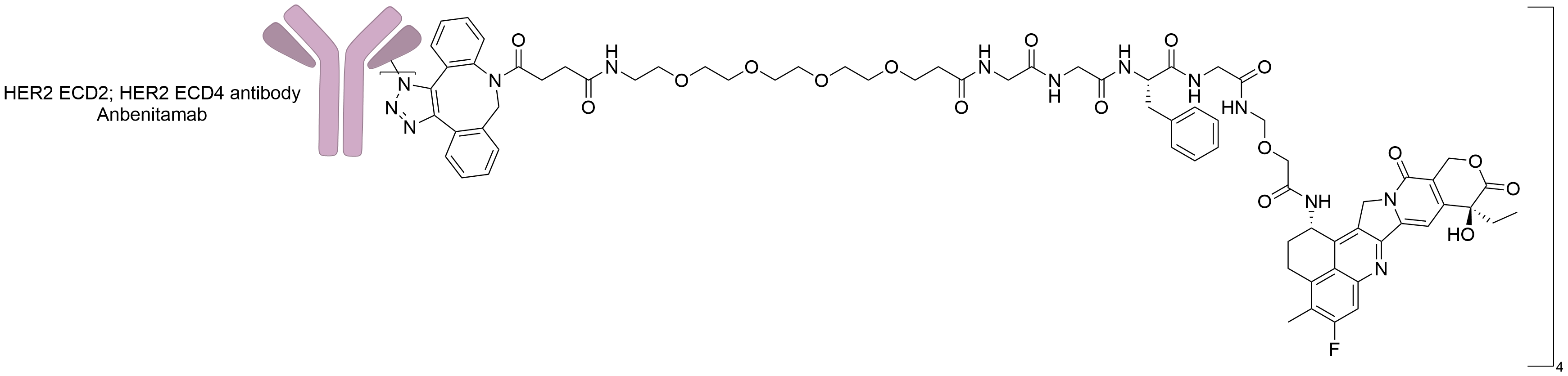
|
|||||
| Antibody Name |
Anbenitamab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2 ECD2); Receptor tyrosine-protein kinase erbB-2 (HER2 ECD4)
|
Antigen Info | ||||
| Payload Name |
DX-8951 derivative (DXd)
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
DBCO-PEG4-Mc-Gly-Gly-Phe-Gly
|
Linker Info | ||||
| Conjugate Type |
Enzymatic Catalysis
|
|||||
