Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0EZVZS
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
BYON-4413
|
|||||
| Synonyms |
BYON 4413; BYON-4413; BYON4413
Click to Show/Hide
|
|||||
| Organization |
Byondis BV
|
|||||
| Drug Status |
Phase 1
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
Undisclosed
|
|||||
| Structure |
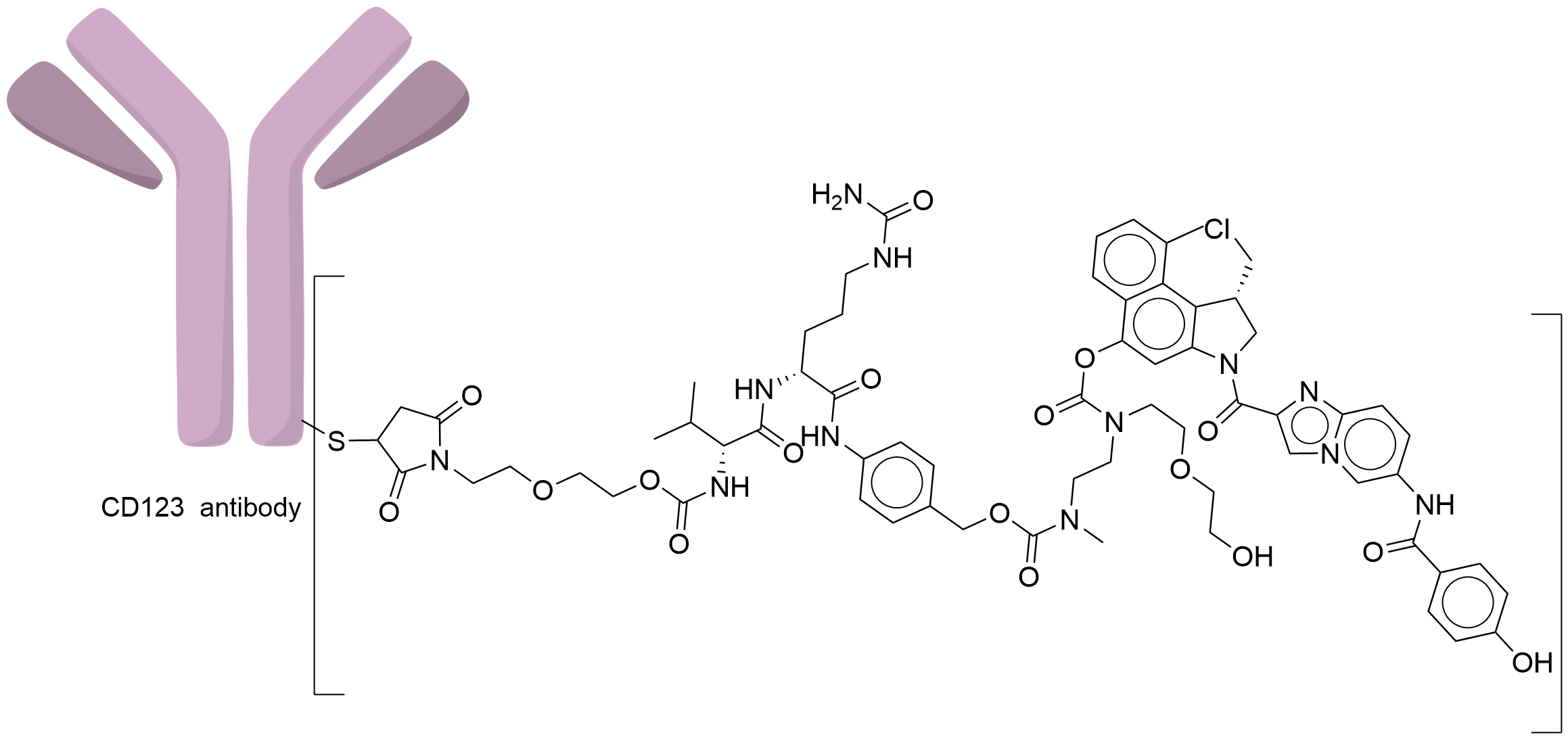
|
|||||
| Antibody Name |
A humanized anti-CD123 IgG1 antibody
|
Antibody Info | ||||
| Antigen Name |
Interleukin-3 receptor subunit alpha (IL3RA)
|
Antigen Info | ||||
| Payload Name |
seco-DUBA
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-PEG2-Val-Cit-PABA-Cyclization Spacer
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
duocarmazine
|
|||||
