Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ENYRQ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
DB-1303
|
|||||
| Synonyms |
BNT323; DB 1303; DB-1303; DB1303
Click to Show/Hide
|
|||||
| Organization |
Duality Biologics; BioNTech SE
|
|||||
| Drug Status |
Phase 3
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
8
|
|||||
| Structure |
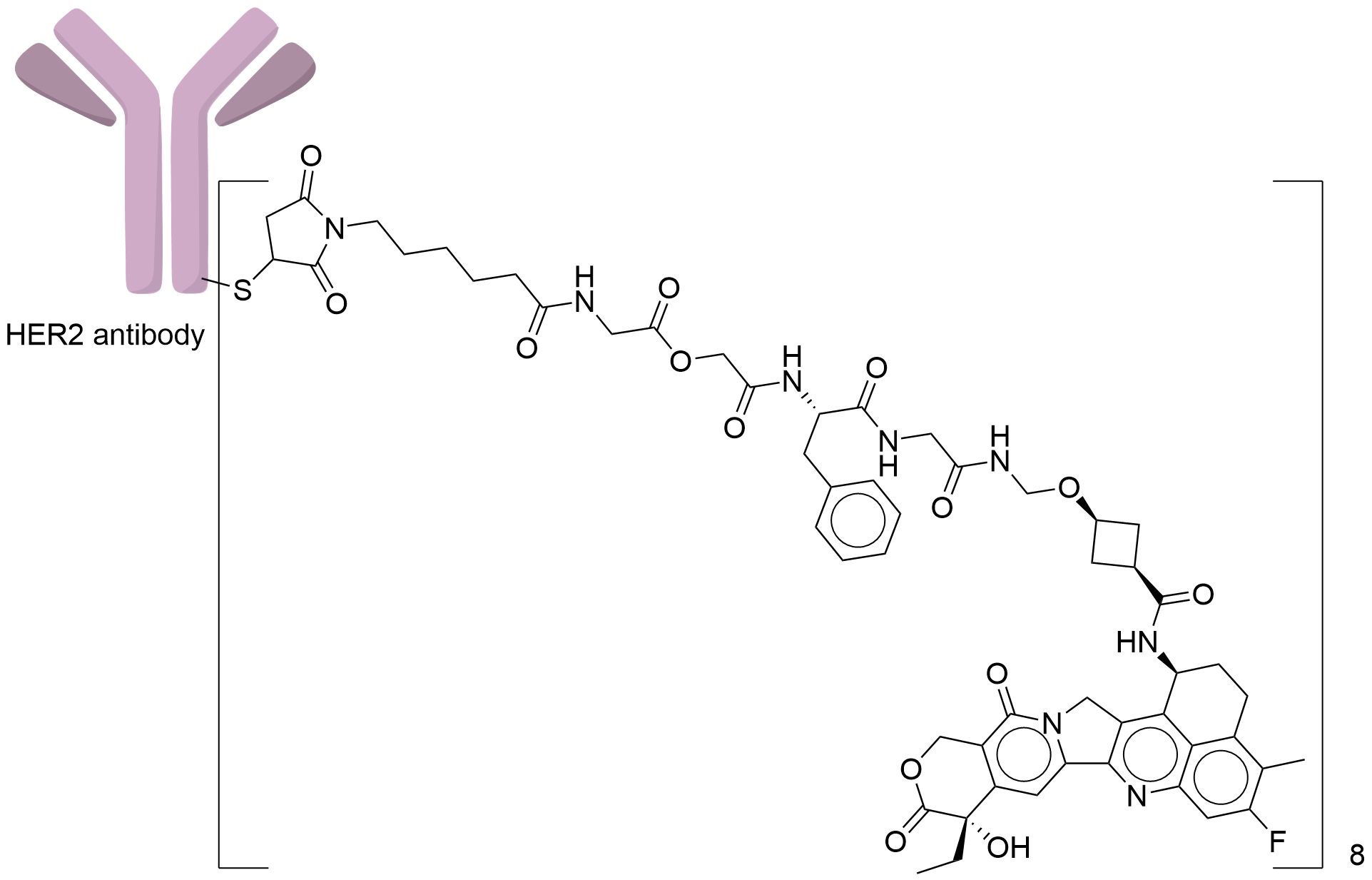
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
P1003
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Mc-Gly-Gly-Phe-Gly
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Special Approval(s) |
Fast track(FDA)
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
44.20
50.00% (HER2-positive BC) 38.50% (HER2-Low BC) 66.70% (CRC) 50.00% (EsC) 50.00% (OC) 33.30% (EC) |
|||
| Patients Enrolled |
Pretreated advanced or metastatic solid tumors; Histologically confirmed HER2-positive or HER2- expressing cancers.
|
||||
| Administration Dosage |
2.20 - 12.00 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05150691 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1/2a, multicenter, open-label, non-randomized first in human study to assess the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of DB-1303 in patients with advanced/metastatic solid tumors. | ||||
References
