Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0EKTUN
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Sacituzumab govitecan
|
|||||
| Brand Name |
Trodelvy
|
|||||
| Synonyms |
GS-0132;IMMU-132;IMMU0132;TROP-2-SN38;hRS7-SN38 antibody drug conjugate;Isactuzumab govitecan
Click to Show/Hide
|
|||||
| Organization |
Immunomedics, Inc.; Gilead Sciences, Inc.;BSP Pharmaceuticals SpA;Everest Medicines (Singapore) Pte. Ltd
|
|||||
| Drug Status |
Approved (FDA): Apr, 2020
|
|||||
| Indication |
In total 22 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
7.6
|
|||||
| Structure |
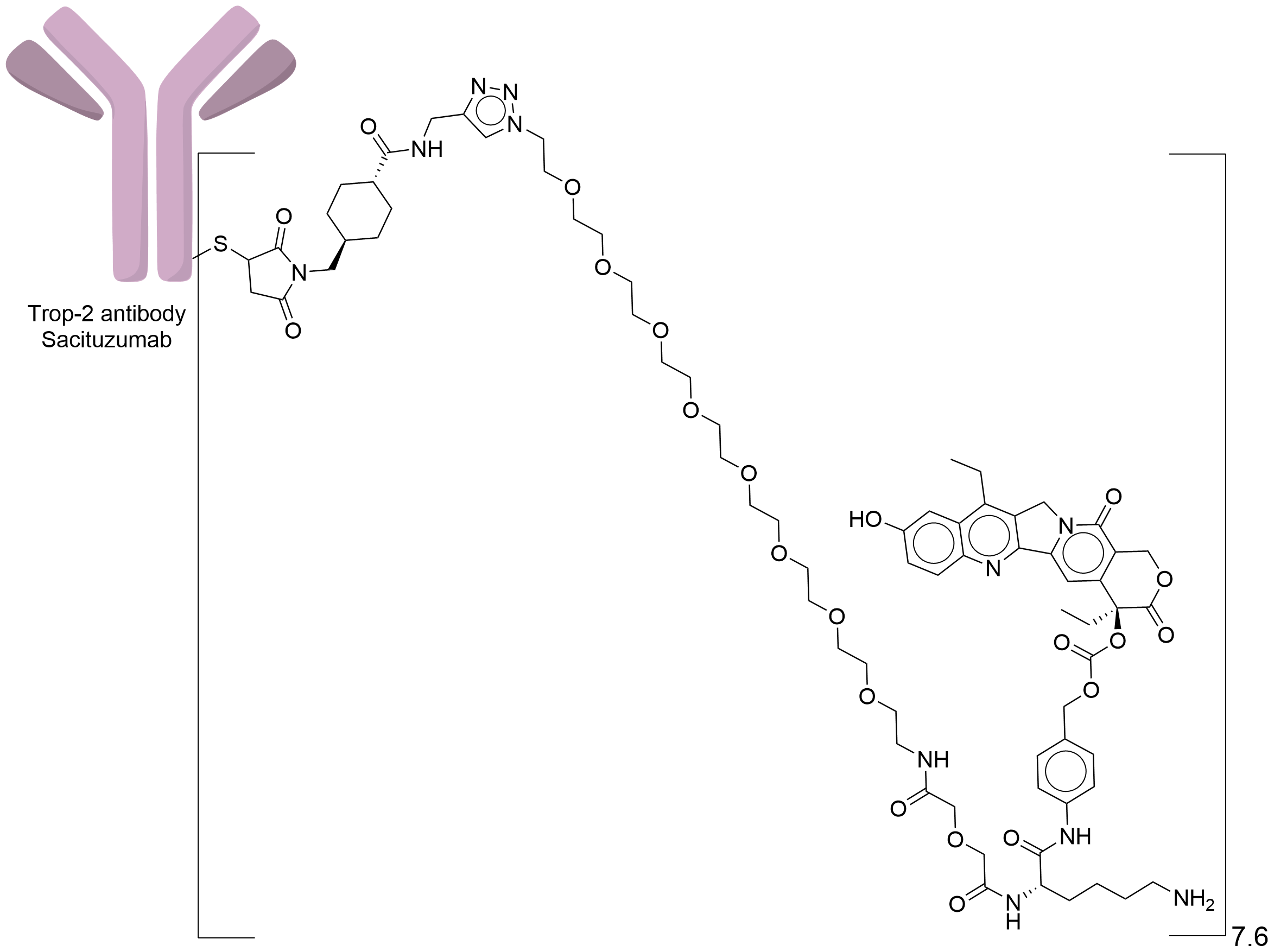
|
|||||
| Antibody Name |
Sacituzumab
|
Antibody Info | ||||
| Antigen Name |
Tumor-associated calcium signal transducer 2 (TROP2)
|
Antigen Info | ||||
| Payload Name |
Active metabolite of irinotecan SN38
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
CL2A
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Combination Type |
Govitecan
|
|||||
| Absorption |
For sacituzumab govitecan (10 mg/kg), mean Cmax was 243.0±45.6 (ug/mL) and mean AUC was 5,210±1,230 (ug x h/mL). For free SN-38, mean Cmax was 127±60 (ng/mL) and mean AUC was 3.90±1.83 (ug x h/mL).
|
|||||
| Distribution |
Sacituzumab govitecan has a mean volume of distribution of 0.045 L/kg. The active element, SN-38, is predominantly attached to the IgG component in serum. After administering 10 mg/kg of sacituzumab govitecan, free SN-38 serum levels were measured at 2.3% after 30 minutes and 4.5% after one day.
|
|||||
| Metabolism |
The metabolism of sacituzumab govitecan has not been extensively studied. The SN-38 moiety is known to undergo O-glucuronidation by UGT1A1, presumably in the liver, and the SN-38 glucuronide metabolite SN-38G is found in the serum of patients undergoing treatment. Sacituzumab govitecan has a mean half-life of 16 hours, while free SN-38 has a mean half-life of 18 hours.
|
|||||
| Elimination |
Renal elimination of SN-38 is known to be minimal, and it is expected that the fecal route will be the major contributor.
|
|||||
| Toxicity |
Patients experiencing an overdose are at an increased risk of severe adverse effects such as neutropenia, diarrhea, hypersensitivity, nausea/vomiting, and other systemic effects related to cytotoxic drugs. FDA black box warning: severe neutropenia and diarrhea may occur.
|
|||||
| Special Approval(s) |
Priority review(FDA); Accelerated approval(FDA); Fast track(FDA); Orphan drug(FDA); Breakthrough therapy(FDA);Priority review(NMPA)
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
| Standard Type | Value | Units | Animal Model (No. of PDX) |
|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) |
2.14
|
nM
|
Endometrioma PDX model (PDX: END(K)265)
|
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
21.00%
|
|||
| Patients Enrolled |
Patients had histologically locally confirmed measurable HR+/HER2 MBC and 2-4 prior systemic chemotherapy regimens for metastatic disease. (Neo)adjuvant therapy for early-stage disease qualified as one of the required prior chemotherapy regimens if the development of unresectable, locally advanced, or metastatic disease occurred within 12 months of therapy (early relapse). Patients must have previously received at least one taxane, at least one anticancer hormonal treatment, and at least one CDK4/6i.
Click to Show/Hide
|
||||
| Administration Dosage |
10 mg/kg intravenously once weekly on day 1 and day 8 every 21 days.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03901339 | Clinical Status | Phase 3 | ||
| Clinical Description | Phase 3 study of sacituzumab govitecan (IMMU-132) versus treatment of physician's choice (tpc) in subjects with hormonal receptor-positive (hr+) human epidermal growth factor receptor 2 (HER2) negative metastatic breast cancer (MBC) who have failed at least two prior chemotherapy regimens. | ||||
| Primary Endpoint |
medium PFS=5.50 months.
|
||||
| Other Endpoint |
medium OS=13.90 months.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
31.43%
|
|||
| Patients Enrolled |
Patients had metastatic triple-negative breast cancer (mTNBC) that had progressed following 2 prior standard chemotherapy regimens (no upper limit) for unresectable, locally advanced, or metastatic disease, and included a taxane (any setting). Per protocol, patients were also eligible after only one prior regimen in the metastatic setting if their disease recurred within 12 months of completing (neo)adjuvant therapy.
Click to Show/Hide
|
||||
| Administration Dosage |
10 mg/kg on days 1 and 8 of 21-day cycles; until progression, unacceptable toxicity, study withdrawal, or death.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02574455 | Clinical Status | Phase 3 | ||
| Clinical Description | Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the phase 3 randomized ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. | ||||
| Primary Endpoint |
PFS=4.60 months for patients without TNBC at initial diagnosis PFS=5.70 months for patients with TNBC at initial diagnosis.
|
||||
| Other Endpoint |
ORR=31.43%.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
35.00%
|
|||
| Patients Enrolled |
Patients with metastatic triple-negative breast cancer that was relapsed or refractory to two or more previous standard chemotherapy regimens (no upper limit) for unresectable, locally advanced or metastatic disease; previous therapy had to include a taxane (for any indication). Patients had to have triple-negative breast cancer according to standard American Society of Clinical OncologyCollege of American Pathologists criteria.
Click to Show/Hide
|
||||
| Administration Dosage |
Sacituzumab govitecan at a dose of 10 mg per kilogram of body weight intravenously on days 1 and 8 of each 21-day cycle.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02574455 | Clinical Status | Phase 3 | ||
| Clinical Description | An international, multi-center, open-label, randomized, phase 3 trial of sacituzumab govitecan versus treatment of physician choice in patients with metastatic triple-negative breast cancer who received at least two prior treatments. | ||||
| Primary Endpoint |
Progression-free survival=5.60 months,(95% CI, 4.30-6.30) with sacituzumab govitecan and 1.70 months (95% CI,1.50 to 2.60) with chemotherapy (hazard ratio for disease progression or death, 0.41; 95% CI, 0.32-0.52).
|
||||
| Other Endpoint |
The percentage of patients with an objective response was 35.00% with sacituzumab govitecan and 5.00% with chemotherapy.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 38.80% | High TROP2 expression (TROP2+++) | ||
| Patients Enrolled |
Histologically or cytologically confirmed metastatic triple negative breast cancer (mTNBC); refractory or relapsing after 2 prior standard chemotherapy for unresectable, locally advanced, or metastatic disease, including a taxane (any setting).
|
||||
| Administration Dosage |
10 mg/kg intravenously on Days 1 and 8 of each 21-day treatment cycle.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04454437 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase IIb, single arm, multicenter trial of sacituzumab govitecan in chinese patients with metastatic triple-negative breast cancer who received at least two prior treatments. | ||||
| Primary Endpoint |
Objective response rate=38.80% (95% CI 28.06-50.30), clinical benefit rate=43.80% (95% CI 32.68-55.30).
|
||||
| Other Endpoint |
Median PFS=5.55 months (95% CI 4.14-N/A).
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
14.00%
|
|||
| Patients Enrolled |
Patients 18 years of age with mSCLC who had relapsed or were refractory to at least one prior standard line of therapy for metastatic disease, and with measurable tumors by CT, were enrolled. They were required to have Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, adequate bone marrow, hepatic and renal function, and other eligibility as described in the phase I trial (25). Previous therapy had to be completed at least 2 weeks before enrollment.
Click to Show/Hide
|
||||
| Administration Dosage |
Either 8 or 10 mg/kg i.v. on days 1 and 8 of 21-day cycles.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01631552 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of IMMU-132 (hRS7-SN38 antibody drug conjugate) in patients with epithelial cancer. | ||||
| Primary Endpoint |
OrR=14.00% (17.00% for 10 mg/kg group).
|
||||
| Other Endpoint |
The median response duration=5.70 months; the clinical benefit rate (CBR>4 months), 34.00%; median PFS=3.70 months; median OS=7.50 months.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
17.70% (small-cell lung cancer)
22.20% (endometrial cancer) 9.10% (castrate-resistant prostate cancer) |
|||
| Patients Enrolled |
Patients were >18 years of age with metastatic cancer [cervical, clear-cell renal, CRC, epithelial ovarian, endometrial, esophageal, gastric, hepatocellular, CRPC, pancreatic ductal adenocarcinoma, squamous cell head and neck, thyroid, urothelial (UC) cancer; glioblastoma multiforme; mTNBC or non-mTNBC; and SCLC or NSCLC] who had relapsed after or were refractory to at least one prior standard therapeutic regimen.
Click to Show/Hide
|
||||
| Administration Dosage |
Intravenous 8, 10, 12, or 18 mg/kg on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01631552 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of IMMU-132 (hRS7-SN38 antibody drug conjugate) in patients with epithelial cancer. | ||||
| Primary Endpoint |
Safety and pharmacokinetic parameters with investigator-evaluated objective response rate.
|
||||
| Other Endpoint |
Efficacy endpoints included: ORR, which included both confirmed partial response (PR) and complete response (CR).
|
||||
| Experiment 7 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
19.00%
|
|||
| Patients Enrolled |
Patients 18 years with mNSCLC who had measurable disease and progressed after at least one line of therapy for stage IV disease were enrolled. Requirements included Eastern Cooperative Oncology Group performance status 0 or 1, adequate bone marrow and hepatic and renal function, and other eligibility criteria as described in the phase I trial. Prior systemic therapy had to be completed at least 4 weeks before enrollment.
Click to Show/Hide
|
||||
| Administration Dosage |
Doses of 8 or 10 mg/kg were given on days 1 and 8 of 21-day cycles; intravenously.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01631552 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of IMMU-132 (hRS7-SN38 antibody drug conjugate) in patients with epithelial cancer. | ||||
| Primary Endpoint |
Safety and objective response rate (ORR).
|
||||
| Other Endpoint |
Progression-free survival and overall survival.
|
||||
| Experiment 8 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
30.00%
|
|||
| Patients Enrolled |
Patients 18 years of age who had mTNBC refractory to or relapsed after at least one standard line of therapy since diagnosis and measurable disease by computed tomography scan (or magnetic resonance imaging). patients had an Eastern Cooperative Oncology Group performance status of 0 or 1, adequate bone marrow, hepatic and renal function, and prior toxicities at study entry of grade 1 by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03. Patients with brain metastasis were excluded, unless treated and without progression, and were not receiving high-dose corticosteroids for at least 4 weeks.
Click to Show/Hide
|
||||
| Administration Dosage |
10 mg/kg starting dose on days 1 and 8 of 21-day repeated cycles; intravenously; eight cycles.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01631552 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of IMMU-132 (hRS7-SN38 antibody drug conjugate) in patients with epithelial cancer. | ||||
| Primary Endpoint |
Safety and objective response rate.
|
||||
| Other Endpoint |
Progression-free survival and overall survival.
|
||||
| Experiment 9 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
33.30%
|
|||
| Patients Enrolled |
Metastatic triple-negative breast cancer.
|
||||
| Administration Dosage |
10 mg per kilogram intravenously on days 1 and 8 of each 21-day cycle until disease progression or unacceptable toxic effects.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01631552 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of IMMU-132 (hRS7-SN38 antibody drug conjugate) in patients with epithelial cancer. | ||||
| Primary Endpoint |
Objective response rate=33.3% (95% confidence interval [CI].
|
||||
| Other Endpoint |
The median duration of response=7.70 months (95% CI, 4.90 to 10.08),clinical benefit rate = 45.40%.
|
||||
| Experiment 10 Reporting the Activity Date of This ADC | [10] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01631552 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of IMMU-132 (hRS7-SN38 antibody drug conjugate) in patients with epithelial cancer. | ||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.14 nM
|
|||
| Method Description |
Antibody-dependent cell cytotoxicity (ADCC) against Trop-2-positive and Trop-2-negative EC cell lines was measured in vitro,compared to control ADC (P = 0.014 and P = 0.005).
|
||||
| In Vivo Model | Endometrioma PDX model (PDX: END(K)265) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.18-0.26 nM
|
|||
| Method Description |
Two Trop-2 positive (CVX-8, ADX-3), and one Trop-2 negative (ADX-2) cell lines were used. A cell line with a strong Trop-2 expression (CVX-8) was used to test in vivo antitumor activity in xenografts models,in vitro experiments.
|
||||
| In Vitro Model | Primary cervical cancer | Primary cervical cancer cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.60 nM
|
|||
| Method Description |
The inhibitory activity of sacituzumab govitecan govitecan against USC with heterogeneous Trop-2 expression was tested by admixing ARK2 USC cells (i.e., high Trop-2 expression) compared to control ADC (p< 0.05),in vitro.
|
||||
| In Vitro Model | Endometrial serous adenocarcinoma | USPC-ARK-2 cells | CVCL_IV73 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.02 nM
|
|||
| Method Description |
Briefly, cells were plated into 96-well clear, flat-bottomed plates. The immunoconjugate reconstituted in media was diluted with media to a final concentration of range of 0.004 to 250 nM in the wells. Plates were incubated in humidified chamber for 96 h 37°C/5% CO2. Growth inhibition was measured as a percent of growth relative to untreated cells.
Click to Show/Hide
|
||||
| In Vitro Model | Colon adenocarcinoma | COLO 205 cells | CVCL_0218 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.44 nM
|
|||
| Method Description |
Briefly, cells were plated into 96-well clear, flat-bottomed plates. The immunoconjugate reconstituted in media was diluted with media to a final concentration of range of 0.004 to 250 nM in the wells. Plates were incubated in humidified chamber for 96 h 37°C/5% CO2. Growth inhibition was measured as a percent of growth relative to untreated cells.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.86 nM
|
|||
| Method Description |
Briefly, cells were plated into 96-well clear, flat-bottomed plates. The immunoconjugate reconstituted in media was diluted with media to a final concentration of range of 0.004 to 250 nM in the wells. Plates were incubated in humidified chamber for 96 h 37°C/5% CO2. Growth inhibition was measured as a percent of growth relative to untreated cells.
Click to Show/Hide
|
||||
| In Vitro Model | Prostate carcinoma | PC-3 cells | CVCL_0035 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.50 nM
|
|||
| Method Description |
Briefly, cells were plated into 96-well clear, flat-bottomed plates. The immunoconjugate reconstituted in media was diluted with media to a final concentration of range of 0.004 to 250 nM in the wells. Plates were incubated in humidified chamber for 96 h 37°C/5% CO2. Growth inhibition was measured as a percent of growth relative to untreated cells.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | Capan-1 cells | CVCL_0237 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [15] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.90 nM
|
|||
| Method Description |
The inhibitory activity of sacituzumab govitecan govitecan against USC with heterogeneous Trop-2 expression was tested by admixing ARK2 USC cells (i.e., high Trop-2 expression) compared to control ADC (p< 0.05),in vitro.
|
||||
| In Vitro Model | Endometrial serous adenocarcinoma | USPC-ARK-2 cells | CVCL_IV73 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.19 nM
|
|||
| Method Description |
Briefly, cells were plated into 96-well clear, flat-bottomed plates. The immunoconjugate reconstituted in media was diluted with media to a final concentration of range of 0.004 to 250 nM in the wells. Plates were incubated in humidified chamber for 96 h 37°C/5% CO2. Growth inhibition was measured as a percent of growth relative to untreated cells.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.61 nM
|
|||
| Method Description |
Briefly, cells were plated into 96-well clear, flat-bottomed plates. The immunoconjugate reconstituted in media was diluted with media to a final concentration of range of 0.004 to 250 nM in the wells. Plates were incubated in humidified chamber for 96 h 37°C/5% CO2. Growth inhibition was measured as a percent of growth relative to untreated cells.
Click to Show/Hide
|
||||
| In Vitro Model | Lung squamous cell carcinoma | SK-MES-1 cells | CVCL_0630 | ||
References
