Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0EDBXG
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Lintuzumab Ac-225
|
|||||
| Synonyms |
225Ac-HuM-195; 225Ac-HuM195; 225Ac-lintuzumab; AC225 MOAB M195; AC225-MOAB-M195; Ac-225-Lintuzumab; Ac225 monoclonal antibody M195; Actimab-A; Actimab-M; Actimab-MDS; Actinium (225ac) lintuzumab satetraxetan; Actinium AC 225 lintuzumab; Actinium AC-225 lintuzumab; Actinium-225 (225Ac)-Lintuzumab; Actinium-225-labeled humanized anti-cd33 monoclonal antibody HUM195; Actinium-225-labelled HuM195; HUM-195 AC-225; HuM195-Ac-225; Lintuzumab Ac-225; Lintuzumab actinium Ac-225; Lintuzumab satetraxetan AC-225
Click to Show/Hide
|
|||||
| Organization |
PDL BioPharma, Inc.; Actinium Pharmaceuticals, Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
Undisclosed
|
|||||
| Structure |
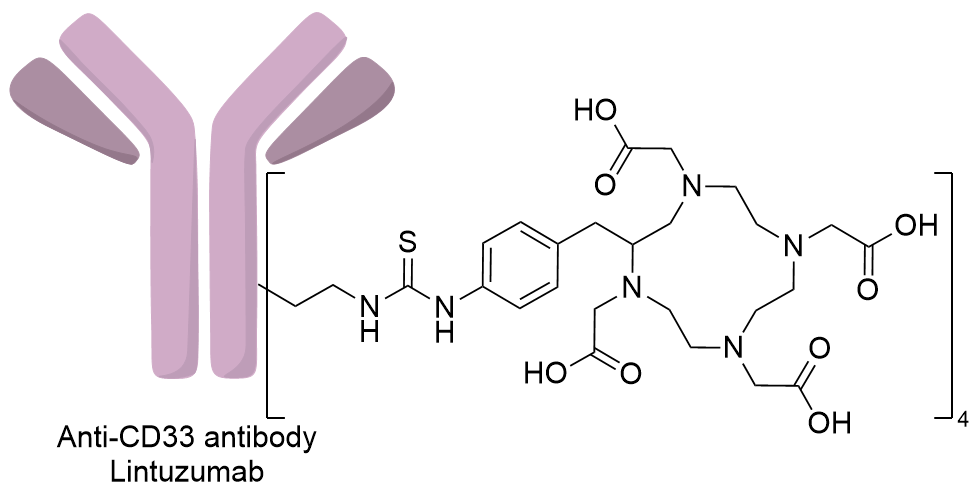
|
|||||
| Antibody Name |
Lintuzumab
|
Antibody Info | ||||
| Antigen Name |
Myeloid cell surface antigen CD33 (CD33)
|
Antigen Info | ||||
| Payload Name |
AC225
|
Payload Info | ||||
| Linker Name |
DOTA-P-toluene isothiocyanate
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Special Approval(s) |
Orphan drug(FDA)
|
|||||
| Puchem SID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
67.00%
|
|||
| Patients Enrolled |
Patients with more than 25% of leukemic blasts must have been CD33 positive by flow cytometry.
|
||||
| Administration Dosage |
Induction consisted of G-CSF, 300 mg/d, given D1-6, cladribine 5 mg/m2, given D2-6, cytarabine 2 ug/m2, given D2-6, and mitoxantrone 10 mg/m2, given D2-4. Lintuzumab Ac225 was administered as a single dose on either day 7, 8, or 9 with a dose of 0.25 uCi/kg, 0.50 uCi/kg, or 0.75 uCi/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03441048 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of lintuzumab-Ac225 in combination with CLAG-M chemotherapy in patients with relapsed/refractory acute myeloid leukemia. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03932318 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of venetoclax and azacitidine and lintuzumab-Ac225 in patients with refractory or relapsed AML. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03867682 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of venetoclax and lintuzumab-Ac225 in patients with refractory or relapsed AML. | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02998047 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of lintuzumab-Ac225 in patients with refractory multiple myeloma. | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02575963 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of lintuzumab-Ac225 in older patients with untreated acute myeloid leukemia. | ||||
References
