Linker Information
General Information of This Linker
| Linker ID |
LIN0PWYIO
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
DOTA-P-toluene isothiocyanate
|
|||||
| Linker Type |
Chelating agent
|
|||||
| Antibody-Linker Relation |
Uncleavable
|
|||||
| Structure |
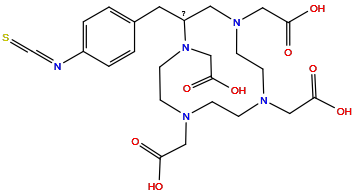
|
|||||
| Formula |
C24H33N5O8S
|
|||||
| Isosmiles |
O=C(O)CN1CCN(CC(=O)O)CCN(CC(=O)O)C(Cc2ccc(N=C=S)cc2)CN(CC(=O)O)CC1
|
|||||
| InChI |
InChI=1S/C24H33N5O8S/c30-21(31)13-26-5-6-27(14-22(32)33)9-10-29(16-24(36)37)20(12-28(8-7-26)15-23(34)35)11-18-1-3-19(4-2-18)25-17-38/h1-4,20H,5-16H2,(H,30,31)(H,32,33)(H,34,35)(H,36,37)
|
|||||
| InChIKey |
UDOPJKHABYSVIX-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
551.622
|
Polar area
|
174.52
|
||
|
Complexity
|
38
|
xlogp Value
|
-0.108
|
|||
|
Heavy Count
|
38
|
Rot Bonds
|
11
|
|||
|
Hbond acc
|
10
|
Hbond Donor
|
4
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Lintuzumab Ac-225 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
67.00%
|
|||
| Patients Enrolled |
Patients with more than 25% of leukemic blasts must have been CD33 positive by flow cytometry.
|
||||
| Administration Dosage |
Induction consisted of G-CSF, 300 mg/d, given D1-6, cladribine 5 mg/m2, given D2-6, cytarabine 2 ug/m2, given D2-6, and mitoxantrone 10 mg/m2, given D2-4. Lintuzumab Ac225 was administered as a single dose on either day 7, 8, or 9 with a dose of 0.25 uCi/kg, 0.50 uCi/kg, or 0.75 uCi/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03441048 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1 study of lintuzumab-Ac225 in combination with CLAG-M chemotherapy in patients with relapsed/refractory acute myeloid leukemia.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03932318 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1/2 study of venetoclax and azacitidine and lintuzumab-Ac225 in patients with refractory or relapsed AML.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03867682 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1/2 study of venetoclax and lintuzumab-Ac225 in patients with refractory or relapsed AML.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02998047 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1 study of lintuzumab-Ac225 in patients with refractory multiple myeloma.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02575963 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1/2 study of lintuzumab-Ac225 in older patients with untreated acute myeloid leukemia.
|
||||
References
