Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0CXIUK
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
T-CpHK-Tet-ADC
|
|||||
| Synonyms |
T-CpHK Tet ADC
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.7
|
|||||
| Structure |
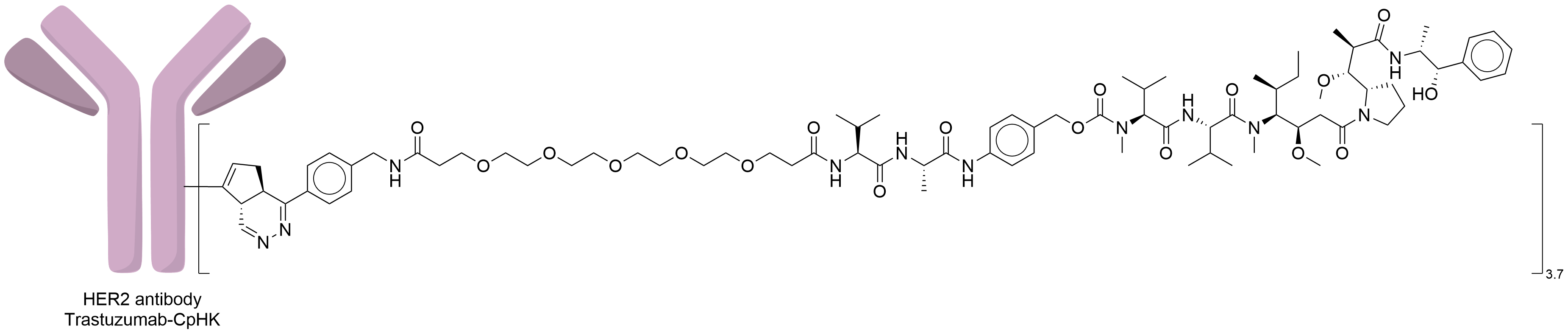
|
|||||
| Antibody Name |
Trastuzumab-CpHK
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Tetrazine-PEG5-Val-Ala-PABC
|
Linker Info | ||||
| Conjugate Type |
CpHK was incorporated at the antibody heavy chain, replacing the amino acid residue K274 to yield reactive antibodies bearing cyclopentadiene groups, tetrazinecyclopentadiene conjugating, DielsAlder (DA) reaction.
|
|||||
| Combination Type |
Tetrazine-PEG5-Val-Ala-PABC-MMAE
|
|||||
General Information of The Activity Data Related to This ADC
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.30 nM | High HER2 expression (HER2+++) | ||
| Method Description |
ADCs were subjected to cytotoxicity assays to confirm potency toward cell lines with high and low HER2 expression.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.40 nM | High HER2 expression (HER2+++) | ||
| Method Description |
ADCs were subjected to cytotoxicity assays to confirm potency toward cell lines with high and low HER2 expression.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 68.00 nM | Moderate HER2 expression (HER2++) | ||
| Method Description |
ADCs were subjected to cytotoxicity assays to confirm potency toward cell lines with high and low HER2 expression.
|
||||
| In Vitro Model | Invasive breast carcinoma | ZR-75-1 cells | CVCL_0588 | ||
References
