Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0COMTY
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Telisotuzumab vedotin
|
|||||
| Brand Name |
Emrelis
|
|||||
| Synonyms |
ABBV 399; ABBV-399; ABBV399; ABT 399; ABT-399; Teliso-V; Teliso-V (ABBV-399); Telisotuzumab vedotin
Click to Show/Hide
|
|||||
| Organization |
AbbVie, Inc.; BSP Pharmaceuticals SpA
|
|||||
| Drug Status |
Approved (FDA): May, 2025
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.1
|
|||||
| Structure |
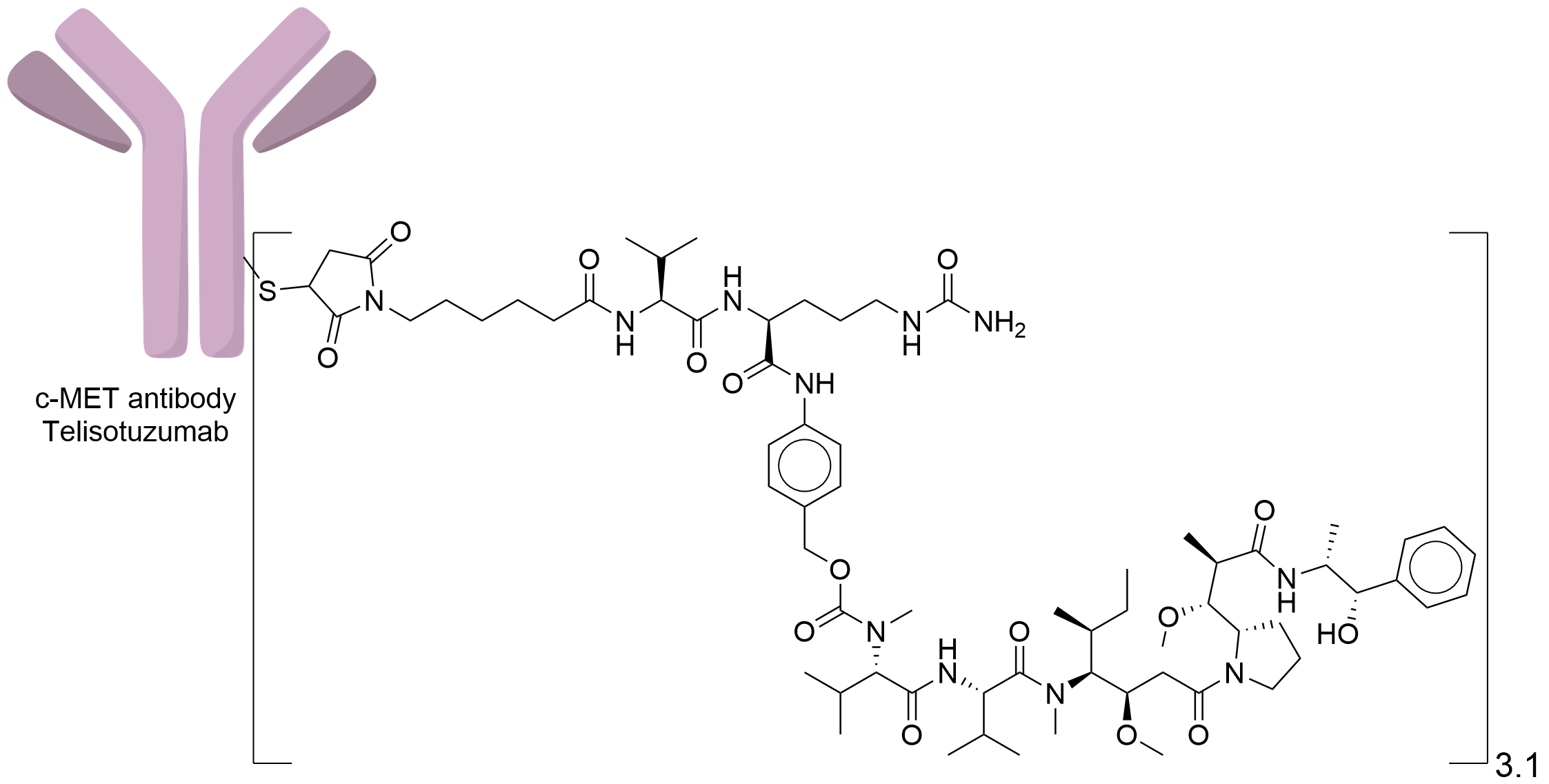
|
|||||
| Antibody Name |
Telisotuzumab
|
Antibody Info | ||||
| Antigen Name |
Hepatocyte growth factor receptor (MET)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
vedotin
|
|||||
| Special Approval(s) |
Breakthrough therapy(FDA)
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
7.40%
|
|||
| Patients Enrolled |
Advanced non-small cell lung cancer (NSCLC).
|
||||
| Administration Dosage |
Teliso-V Q2W (1.60, 1.90, or 2.20 mg/kg, intravenous) with nivolumab (3 mg/kg, or 240 mg, or per locally approved label, intravenously).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02099058 | Clinical Status | Phase 1 | ||
| Clinical Description | A multicenter, phase 1/1b, open-label, dose-escalation study of ABBV-399, an antibody drug conjugate, in subjects with advanced solid tumors. | ||||
| Primary Endpoint |
Most patients (97.30%, n=36) experienced one or more TEAE, with 23 (62.16%) reporting TEAEs grades 3 or higher. TEAEs considered possibly related to Teliso-V were reported in 78.38% (n=29) of patients; 32.43% (n=12) were grade greater than or equal to 3.
|
||||
| Other Endpoint |
Combination therapy with Teliso-V plus nivolumab was well tolerated in patients with c-Met-+NSCLC with limited antitumor activity. The ORR was 7.40% (95% CI: 0.90-24.30), with two patients (PD-L1+, n =1; PD-L1-, n=1) having a confirmed PR.Overall, 66.67% of patients (16 of 24) had evidence of tumor size reduction; three (12.5%) reported a greater than 30% reduction in target lesion. The overall median PFS (95% CI) was 7.20 months (3.30-8.90); 7.20 months(1.50-not reached [NR]) for PD-L1 patients, 4.50 months(1.50-NR) for PD-L1- patients, and NR (2.00-NR) for PD-L1-unk patients. The objective response rate was 7.40%, with two patients having a confirmed partial response. Overall median progression-free survival was 7.20 months.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
23.00% (all)
28.00% (in once every 2 weeks cohorts all) 18.00% (in once every 3 weeks cohorts) 18.00% (nonsquamous NSCLC) 31.00% (in once every 2 weeks cohorts nonsquamous NSCLC) 6.00% (in once every 3 weeks cohorts nonsquamous NSCLC) 43.00% (squamous NSCLC) |
|||
| Patients Enrolled |
Non-small cell lung cancer (NSCLC) and c-Met H-score 150 (c-Met+) or MET amplification/exon 14 skipping mutations.
|
||||
| Administration Dosage |
Intravenously once every 3 weeks (0.15-3.30 mg/kg) or once every 2 weeks (1.60-2.20 mg/kg).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02099058 | Clinical Status | Phase 1 | ||
| Clinical Description | A multicenter, phase 1/1b, open-label, dose-escalation study of ABBV-399, an antibody drug conjugate, in subjects with advanced solid tumors. | ||||
| Primary Endpoint |
Four objective responses (ORR = 26.70%; 95% CI, 7.80-55.10) were observed in this subgroup, 3 in once every 2 weeks (ORR = 43.00%; 95% CI, 9.90-81.60), and 1 in once every 3 weeks (ORR = 13.00%; 95% CI, 0.30-52.70).
|
||||
| Other Endpoint |
The median PFS in once every 2 weeks cohorts was 8.00 months (range, 1.20-9.10) and the median treatment duration was 19.60 weeks (range, 0.10-60.10).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
30.55% (for all efficacy-evaluable patients)
32.10% (for EGFR-M+ patients) 52.60% (of EGFR-M+ patients, those who were c-Met high) |
|||
| Patients Enrolled |
Advanced non-small cell lung cancer (measurable per Response Evaluation Criteria in Solid Tumors v1.1) not amenable to resection or other approved therapies until disease progression, death, or withdrawal of consent.
|
||||
| Administration Dosage |
Teliso-V (2.70 mg/kg once every 21 days) plus erlotinib (150 mg once daily) until disease progression, death, or withdrawal of consent.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02099058 | Clinical Status | Phase 1 | ||
| Clinical Description | A multicenter, phase 1/1b, open-label, dose-escalation study of ABBV-399, an antibody drug conjugate, in subjects with advanced solid tumors. | ||||
| Primary Endpoint |
OrR for all efficacy-evaluable patients was 30.55% (11/36; 95% CI, 16.30 to 48.10), and DCR was 86.11% (31/36; 95% CI, 70.5 to 95.3). Median PFS for all efficacy-evaluable patients was 5.90 months (95% CI, 2.80 to not reached [NR]).
|
||||
| Other Endpoint |
For EGFR-M+ patients (n = 28), ORR was 32.14% (9/28; 95% CI, 15.90 to 52.40), with one CR (3.57%) and eight PR (28.57%). DCR was 85.71% (24/28; 95% CI, 67.30 to 96.00) and median PFS was 5.90 months (95% CI, 2.80 to NR). Median PFS was 3.70 months (95% CI, 1.40 to NR) for T790M+ patients, compared with 6.80 months (95% CI, 4.30 to NR) for non-T790M+ patients. Of EGFR-M+ patients, those who were c-Met high (n = 15) had an ORR of 52.60%. Median PFS was 6.80 months for non-T790M+ and for those whose T790M status was unknown, versus 3.70 months for T790M+.
Click to Show/Hide
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
30.60% (for all efficacy-evaluable patients)
32.18% (for EGFR-M+ patients) 52.60% (of EGFR-M+ patients, those who were c-Met high) |
|||
| Patients Enrolled |
Advanced non-small cell lung cancer (measurable per Response Evaluation Criteria in Solid Tumors v1.1) not amenable to resection or other approved therapies until disease progression, death, or withdrawal of consent.
|
||||
| Administration Dosage |
Teliso-V (2.70 mg/kg once every 21 days) plus erlotinib (150 mg once daily) until disease progression, death, or withdrawal of consent.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02099058 | Clinical Status | Phase 1 | ||
| Clinical Description | A multicenter, phase 1/1b, open-label, dose-escalation study of ABBV-399, an antibody drug conjugate, in subjects with advanced solid tumors. | ||||
| Primary Endpoint |
Median PFS=5.90 months (95% CI, 2.80 to not reached). ORR for EGFR-M+ patients = 32.18% (n=28). EGFR-M+ patients ORR = 52.60%.
|
||||
| Other Endpoint |
Median PFS=6.80 months for non-T790M+.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
71.70% (plus lenalidomide)
70.60% (plus pomalidomide) |
|||
| Patients Enrolled |
Relapsed or refractory multiple myeloma, and ECOG performance status or Zubrod score of 2 or below, received indatuximab ravtansine with lenalidomide and dexamethasone (indatuximab ravtansine plus lenalidomide) had failure of at least one previous therapy.
|
||||
| Administration Dosage |
Intravenously on days 1, 8, and 15 of each 28-day cycle in dose of 100 mg/m2 plus lenalidomide or pomalidomide and dexamethasone.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01638936 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2a multi-dose escalation study of BT062 in combination with lenalidomide or pomalidomide and dexamethasone in subjects with relapsed or relapsed/refractory multiple myeloma. | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
75.00%
|
|||
| Patients Enrolled |
MA advanced GEC.
|
||||
| Administration Dosage |
15 mg/kg IV, once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01472016 | Clinical Status | Phase 1 | ||
| Clinical Description | A multi-center, phase 1/1b, open-label, dose escalation study of ABT-700, a monoclonal antibody in subjects with advanced solid tumors. | ||||
| Primary Endpoint |
Among these patients, three achieved a partial response and one had progressive disease as best response (ORR=75.00%). The duration of disease control in responders ranged from 18-27 weeks and the median duration of response was 16.10 weeks. The median progression- free survival in MET-amplified patients was 17.90 weeks.
|
||||
| Experiment 7 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01915472 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase 2 study of IMMU 130 (hmn-14-SN38 antibody drug conjugate) in patients with metastatic colorectal cancer. | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [7] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01001442 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1/2a multi-dose escalation study to evaluate maximum tolerated dose (MTD), pharmacokinetics (PK), safety and efficacy of BT062 in subjects with relapsed or relapsed/refractory multiple myeloma. | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [8] | ||||
| Patients Enrolled |
Nonsmall-cell lung cancer (NSCLC) with c-Metoverexpressing tumors (c-Met positive; immunohistochemistry membrane H-score 150).
|
||||
| Administration Dosage |
Teliso-V was administered by intravenous (IV) infusion to groups of three to six patients who were enrolled in eight-dose cohorts for dosing at 0.15 to 3.30 mg/kg on day 1, once every 21 days, or until disease progression or unacceptable toxicity.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02099058 | Clinical Status | Phase 1 | ||
| Clinical Description | A multicenter, phase 1/1b, open-label, dose-escalation study of ABBV-399, an antibody drug conjugate, in subjects with advanced solid tumors. | ||||
| Primary Endpoint |
No formal MTD was identified.
|
||||
| Experiment 10 Reporting the Activity Date of This ADC | [9] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01605318 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of once or twice weekly IMMU-130 (hMN-14-SN38, antibody-drug conjugate) in patients with colorectal cancer. | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [10] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01270698 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of IMMU-130 (hmn-14-SN38 antibody drug conjugate) in patients with colorectal cancer. | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [11] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00723359 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 dose escalation study to evaluate maximum tolerated dose (MTD), pharmacokinetics (PK), and safety of BT062 in subjects with relapsed or relapsed/refractory multiple myeloma. | ||||
References
