Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0AVDEL
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Trastuzumab-PNUEDAGly5
|
|||||
| Synonyms |
Trastuzumab PNU EDA Gly5; Tras PNU EDA Gly5; Tras-PNUEDAGly5
Click to Show/Hide
|
|||||
| Organization |
NBE Therapeutics AG
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.9
|
|||||
| Structure |
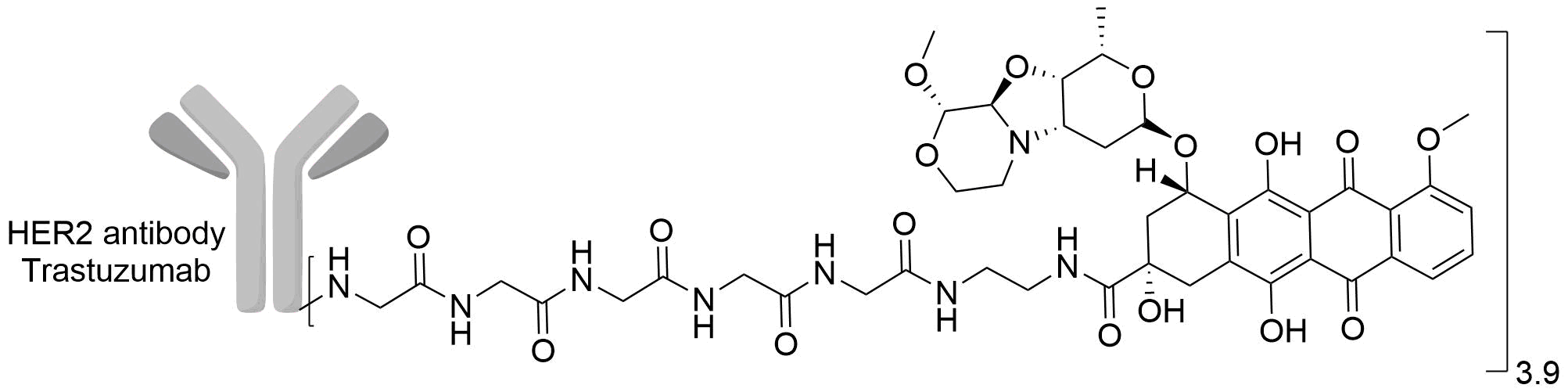
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
PNU-159682
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 2-alpha (TOP2A)
|
Target Info | ||||
| Linker Name |
LPETG-Gly5-EDA
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through nucleophilic lysines.
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
≈ 100
|
%
|
EMT6 cells (High HER2 expression)
|
Mammary gland malignant neoplasms
|
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
1,000,000 EMT6 mouse breast cancer cells expressing human HER-2 previously determined to be suitable for in vivo growth, were implanted into theright mammary fat pads of female Balb/c mice. Animals were treated onthe same day (day 13) and 7 days later (day 20) byintravenous injection of the reference ADC Kadcyla (15 mg/kg), Trastuzumab-PNU-EDA-Glys (1 mg/kg) or vehicle control.
Click to Show/Hide
|
||||
| In Vivo Model | EMT6 CDX model (Expressing hHER2) | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells (High HER2 expression) | CVCL_1923 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 6.90 ng/mL | High CD30 expression (CD30 +++) | ||
| Method Description |
Dose response of the cytotoxic effects of the indicated ADCs on human Non-Hodgkin lymphoma cell line Karpas-299, and on human Hodgkin lymphoma cell line L428 cells.
|
||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | < 10.00 ug/mL | Low CD30 expression (CD30 +) | ||
| Method Description |
Dose response of the cytotoxic effects of the indicated ADCs on human Non-Hodgkin lymphoma cell line Karpas-299, and on human Hodgkin lymphoma cell line L428 cells.
|
||||
| In Vitro Model | Hodgkin lymphoma | L-428 cells | CVCL_1361 | ||
References
