Payload Information
General Information of This Payload
| Payload ID | PAY0QCAJM |
|||||
|---|---|---|---|---|---|---|
| Name | Saporin |
|||||
| Synonyms |
Ber-H2/saporin; D0A9QL
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
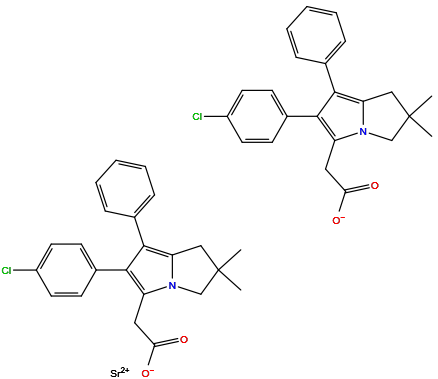
|
|||||
| Formula | C46H42Cl2N2O4Sr |
|||||
| Isosmiles | CC1(CC2=C(C(=C(N2C1)CC(=O)[O-])C3=CC=C(C=C3)Cl)C4=CC=CC=C4)C.CC1(CC2=C(C(=C(N2C1)CC(=O)[O-])C3=CC=C(C=C3)Cl)C4=CC=CC=C4)C.[Sr+2] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/2C23H22ClNO2.Sr/c2*1-23(2)13-19-22(15-6-4-3-5-7-15)21(16-8-10-17(24)11-9-16)18(12-20(26)27)25(19)14-23;/h2*3-11H,12-14H2,1-2H3,(H,26,27);/q;;+2/p-2
|
|||||
| InChIKey |
LRDCMWNTZTVTAN-UHFFFAOYSA-L
|
|||||
| IUPAC Name |
strontium;2-[2-(4-chlorophenyl)-6,6-dimethyl-1-phenyl-5,7-dihydropyrrolizin-3-yl]acetate
|
|||||
| Pharmaceutical Properties | Molecule Weight |
845.4 |
Polar area |
90.1 |
||
Complexity |
531 |
xlogp Value |
. |
|||
Heavy Count |
55 |
Rot Bonds |
6 |
|||
Hbond acc |
4 |
Hbond Donor |
0 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | >1.00E-08 | M |
Jurkat cells
|
T acute lymphoblastic leukemia
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | >1.00E-08 | M |
Raji cells
|
EBV-related Burkitt lymphoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | >1.00E-08 | M |
MOLT-4 cells
|
Adult T acute lymphoblastic leukemia
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | >1.00E-08 | M |
D430B cells
|
Lymphoma
|
Undisclosed | [1] |
| Cell survival rate | 50 | % |
HCT 116 cells
|
Colon cancer
|
[2] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
HB22.7-SAP [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 2.67% (Day 18) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Free hB22.7 (0.6 mg/kg) plus free sap (0.36 mg/kg).
|
||||
| In Vivo Model | Raji NhLcells xenograft model | ||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 13.33% (Day 18) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
HB22.7-sap (1 mg/kg).
|
||||
| In Vivo Model | Raji NhLcells xenograft model | ||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00%
|
High CD22 expression (CD22+++) | ||
| Method Description |
Nude mice were subcutaneously injected with 5 x 106 Raji cells. Twenty-four h later, designated as day 0, mice were randomly separated into three treatment groups (n = 810 per group) consisting of PBS, free HB22.7 (0.6 mg/kg) plus free SAP (0.36 mg/kg) and HB22.7-SAP (1 mg/kg).
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 ng/mL
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of HB22.7-sap exerted a potent cytotoxic effect against all CD22+ NHL cell lines.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma germinal center B-cell type | DoHH2 cells | CVCL_1179 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.70 ng/mL
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of HB22.7-sap exerted a potent cytotoxic effect against all CD22+ NHL cell lines.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.60 ng/mL
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of HB22.7-sap exerted a potent cytotoxic effect against all CD22+ NHL cell lines.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | SU-DHL-4 cells | CVCL_0539 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.40 ng/mL
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of HB22.7-sap exerted a potent cytotoxic effect against all CD22+ NHL cell lines.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 250.00 ng/mL | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of HB22.7-sap exerted a potent cytotoxic effect against all CD22+ NHL cell lines.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 250.00 ng/mL | Negative CD22 expression (CC22-) | ||
| Method Description |
The inhibitory activity of HB22.7-sap exerted a potent cytotoxic effect against all CD22+ NHL cell lines.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
519.00 ng/mL
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of HB22.7-sap exerted a potent cytotoxic effect against all CD22+ NHL cell lines.
|
||||
| In Vitro Model | Mantle cell lymphoma | Granta-519 cells | CVCL_1818 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 ng/mL
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
The in vitro cytotoxicity of HB22.7-SAP was evaluated using an MTS assay. Cells were seeded in 96-well plates at a density of 1 x104 cells/well in 90 uL media. HB22.7-SAP was serially diluted with media and 10 L of each dilution was added to the appropriate well and incubated continuously for 72 h.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | SU-DHL-4 cells | CVCL_0539 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.60 ng/mL
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
The in vitro cytotoxicity of HB22.7-SAP was evaluated using an MTS assay. Cells were seeded in 96-well plates at a density of 1 1 x104 cells/well in 90 uL media. HB22.7-SAP was serially diluted with media and 10 L of each dilution was added to the appropriate well and incubated continuously for 72 h.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma germinal center B-cell type | DoHH2 cells | CVCL_1179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.70 ng/mL
|
High CD22 expression (CD22+++) | ||
| Method Description |
The in vitro cytotoxicity of HB22.7-SAP was evaluated using an MTS assay. Cells were seeded in 96-well plates at a density of 1x104 cells/well in 90 L media. HB22.7-SAP was serially diluted with media and 10 L of each dilution was added to the appropriate well and incubated continuously for 72 h.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.60 ng/mL
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
The in vitro cytotoxicity of HB22.7-SAP was evaluated using an MTS assay. Cells were seeded in 96-well plates at a density of 1x104 cells/well in 90 L media. HB22.7-SAP was serially diluted with media and 10 L of each dilution was added to the appropriate well and incubated continuously for 72 h.
|
||||
| In Vitro Model | Mantle cell lymphoma | Granta-519 cells | CVCL_1818 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.40 ng/mL
|
High CD22 expression (CD22+++) | ||
| Method Description |
The in vitro cytotoxicity of HB22.7-SAP was evaluated using an MTS assay. Cells were seeded in 96-well plates at a density of 1x104 cells/well in 90 L media. HB22.7-SAP was serially diluted with media and 10 L of each dilution was added to the appropriate well and incubated continuously for 72 h.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 2500.00 ng/mL | Negative CD22 expression (CC22-) | ||
| Method Description |
The in vitro cytotoxicity of HB22.7-SAP was evaluated using an MTS assay. Cells were seeded in 96-well plates at a density of 1 x104 cells/well in 90 uL media. HB22.7-SAP was serially diluted with media and 10 uL of each dilution was added to the appropriate well and incubated continuously for 72 h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
ITcetuximab [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.00% (Day 16) | Positive EGFR expression (EGFR +++/++) | ||
| Method Description |
Mice were injected intraperitoneally with 3 mg/kg of ITcetuximab, and 2days later,2 h after the administration of NPe6 (5 mg/kg) via the tail vein, the tumors were irradiated with a 664 nm laser at a dose of 30 J/cm2 from the diode laser unit.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.36 nM
|
High EGFR expression (EGFR +++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 was evaluated from the sigmoid curve obtained using the curvefitting tool of the ImageJ software.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A-431 cells | CVCL_0037 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.77 nM
|
Positive EGFR expression (EGFR +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 was evaluated from the sigmoid curve obtained using the curvefitting tool of the ImageJ software.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Low EGFR expression (EGFR +) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 was evaluated from the sigmoid curve obtained using the curvefitting tool of the ImageJ software.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
5E3-Saporin ADC [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.90 pM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | NCI-H929 cells | CVCL_1600 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.60 pM
|
Positive SEMA4A expression (SEMA4A +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative SEMA4A expression (SEMA4A -) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Chronic myeloid leukemia | K562 cells | CVCL_0004 | ||
Anti-alpha10-SAP [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
69.00 nM
|
Positive ITGA10 expression (ITGA10+++/++) | ||
| Method Description |
Viability analysis of U3046 mg GBM cells after treatment with increasing concentration of anti-alpha10-SAP or anti-ctrl-SAP for 96 h.
|
||||
| In Vitro Model | Glioblastoma | U3046MG cells | CVCL_IR78 | ||
References
