Payload Information
General Information of This Payload
| Payload ID | PAY0KLGQS |
|||||
|---|---|---|---|---|---|---|
| Name | SHR152852 |
|||||
| Synonyms |
SHR152852
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
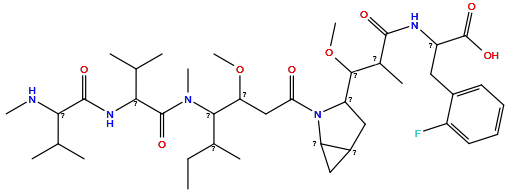
|
|||||
| Formula | C29H44FN3O6 |
|||||
| Isosmiles | [H][C@@]1([C@H](OC)[C@@H](C)C(=O)N[C@@H](Cc2ccccc2F)C(=O)O)C[C@@H]2C[C@@H]2N1C(=O)C[C@@H](OC)[C@@H](NC)[C@@H](C)CC |
|||||
| InChI |
InChI=1S/C29H44FN3O6/c1-7-16(2)26(31-4)24(38-5)15-25(34)33-22-13-19(22)14-23(33)27(39-6)17(3)28(35)32-21(29(36)37)12-18-10-8-9-11-20(18)30/h8-11,16-17,19,21-24,26-27,31H,7,12-15H2,1-6H3,(H,32,35)(H,36,37)/t16-,17+,19-,21-,22-,23-,24+,26-,27+/m0/s1
|
|||||
| InChIKey |
WGMTVOXRADGSPR-KLGFYWOZSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
549.684 |
Polar area |
117.2 |
||
Complexity |
549.3214143 |
xlogp Value |
2.6173 |
|||
Heavy Count |
39 |
Rot Bonds |
15 |
|||
Hbond acc |
6 |
Hbond Donor |
3 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 214.7 | nmol/L |
HCC827 cells
|
Lung adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 283.7 | nmol/L |
Hep-G2 cells
|
Hepatoblastoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 47.7 | nmol/L |
SK-BR-3 cells
|
Breast adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 10.4±5.1 | nM |
MKN45 cells
|
Gastric adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 10.4±5.1 | nM |
MKN45 cells
|
Gastric adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 117.7±2.1 | nM |
Caki-1 cells
|
Clear cell renal cell carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 117.7±2.1 | nM |
Caki-1 cells
|
Clear cell renal cell carcinoma
|
[4] | |
| Half Maximal Inhibitory Concentration (IC50) | 27.1±1.4 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 27.1±1.4 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 28.9±1.3 | nM |
NCI-H1993 cells
|
Lung adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 28.9±1.3 | nM |
NCI-H1993 cells
|
Lung adenocarcinoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 3.1±0.4 | nM |
HCCLM3 cells
|
Adult hepatocellular carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 3.1±0.4 | nM |
HCCLM3 cells
|
Adult hepatocellular carcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 36.2±1.4 | nM |
NCI-N87 cells
|
Gastric tubular adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 36.2±1.4 | nM |
NCI-N87 cells
|
Gastric tubular adenocarcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 77.6±12.1 | nM |
NCI-H441 cells
|
Lung papillary adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 77.6±12.1 | nM |
NCI-H441 cells
|
Lung papillary adenocarcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 89.5±15.2 | nM |
PC-3 cells
|
Prostate carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 89.5±15.2 | nM |
PC-3 cells
|
Prostate carcinoma
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 94.4±3.8 | nM |
Hs 578T cells
|
Invasive breast carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 94.4±3.8 | nM |
Hs 578T cells
|
Invasive breast carcinoma
|
[11] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
SHR-A1403 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03856541 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1, open label, dose escalation study to evaluate the safety, tolerability and pharmacokinetics of SHR-a1403 with intravenous infusion in patients with advanced solid tumors.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.50% (Day 17) | High MET expression (MET+++) | ||
| Method Description |
HCC tumor cells derived from patients (passage 5) were implanted subcutaneously in BALB/c nude mice at an initial tumor size of approximately 30 mm3. In this model,tumor-bearing mice were given vehicle,SHR-A1403 (1 mg/kg),or SHR-A1403 mAb (10 mg/kg) via twice-weekly intravenous injection for two consecutive weeks.
|
||||
| In Vivo Model | Hepatic cancer PDX model (PDX: HCC) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.30% (Day 17) | High MET expression (MET+++) | ||
| Method Description |
HCC tumor cells derived from patients (passage 5) were implanted subcutaneously in BALB/c nude mice at an initial tumor size of approximately 30 mm3. In this model,tumor-bearing mice were given vehicle,SHR-A1403 (3 mg/kg),or SHR-A1403 mAb (10 mg/kg) via twice-weekly intravenous injection for two consecutive weeks.
|
||||
| In Vivo Model | Hepatic cancer PDX model (PDX: HCC) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.50% (Day 17) | High MET expression (MET+++) | ||
| Method Description |
HCC tumor cells derived from patients (passage 5) were implanted subcutaneously in BALB/c nude mice at an initial tumor size of approximately 30 mm3. In this model,tumor-bearing mice were given vehicle,SHR-A1403 (10 mg/kg),or SHR-A1403 mAb (10 mg/kg) via twice-weekly intravenous injection for two consecutive weeks.
|
||||
| In Vivo Model | Hepatic cancer PDX model (PDX: HCC) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 16) | Negative MET expression (MET-) | ||
| Method Description |
Effects of SHR-A1403 were further determined in vivo by assessing the growth of HCC827 and HA1 xenograft tumors. Tumor-bearing mice,established by s.c. inoculation of HCC827 and HA1 cells,were randomized into vehicle,AZD9291 (3 mg/kg single dose,i.g.) and SHR-A1403 (10 mg/kg single dose,i.v.) treatment groups when average tumor volumes reached approximately 100-200 mm3.
Click to Show/Hide
|
||||
| In Vivo Model | Non-small cell lung cancer HCC827 CDX model (CDX: HA1; Afatinib resistant) | ||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells (Afatinib resistant; HA1) | CVCL_2063 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 34.50% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
SHR-A1403 was evaluated in xenograft mice bearing cancer cells with high c-Met expression,including hepatic cancer HCCLM3,lung cancer NCI-H1993,and gastric cancer MKN-45 cells,and the effects were compared with the effects of SHR-A1403 mAb,the free toxin,or their combination. In the HCCLM3 xenograft model,SHR-A1403 was administered at a single dose of 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Hepatic cancer CDX model | ||||
| In Vitro Model | Adult hepatocellular carcinoma | HCCLM3 cells | CVCL_6832 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 41.80% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
SHR-A1403 was evaluated in xenograft mice bearing cancer cells with high c-Met expression,including hepatic cancer HCCLM3,lung cancer NCI-H1993,and gastric cancer MKN-45 cells,and the effects were compared with the effects of SHR-A1403 mAb,the free toxin,or their combination. In the NCI-H1993 xenograft model,SHR-A1403 was administered at a single dose of 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung cancer | Lung cancer cells | Homo sapiens | ||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.80% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
SHR-A1403 was evaluated in xenograft mice bearing cancer cells with high c-Met expression,including hepatic cancer HCCLM3,lung cancer NCI-H1993,and gastric cancer MKN-45 cells,and the effects were compared with the effects of SHR-A1403 mAb,the free toxin,or their combination. In the MKN-45 xenograft model,SHR-A1403 was administered at a single dose of 1 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric cancer | Gastric cancer cells | Homo sapiens | ||
| Experiment 5 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.30% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
SHR-A1403 was evaluated in xenograft mice bearing cancer cells with high c-Met expression,including hepatic cancer HCCLM3,lung cancer NCI-H1993,and gastric cancer MKN-45 cells,and the effects were compared with the effects of SHR-A1403 mAb,the free toxin,or their combination. In the NCI-H1993 xenograft model,SHR-A1403 was administered at a single dose of 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung cancer | Lung cancer cells | Homo sapiens | ||
| Experiment 6 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.30% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
SHR-A1403 was evaluated in xenograft mice bearing cancer cells with high c-Met expression,including hepatic cancer HCCLM3,lung cancer NCI-H1993,and gastric cancer MKN-45 cells,and the effects were compared with the effects of SHR-A1403 mAb,the free toxin,or their combination. In the HCCLM3 xenograft model,SHR-A1403 was administered at a single dose of 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Hepatic cancer CDX model | ||||
| In Vitro Model | Adult hepatocellular carcinoma | HCCLM3 cells | CVCL_6832 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.60% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
SHR-A1403 was evaluated in xenograft mice bearing cancer cells with high c-Met expression,including hepatic cancer HCCLM3,lung cancer NCI-H1993,and gastric cancer MKN-45 cells,and the effects were compared with the effects of SHR-A1403 mAb,the free toxin,or their combination. In the MKN-45 xenograft model,SHR-A1403 was administered at a single dose of 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | MKN45 cells | CVCL_0434 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 89.90% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
SHR-A1403 was evaluated in xenograft mice bearing cancer cells with high c-Met expression,including hepatic cancer HCCLM3,lung cancer NCI-H1993,and gastric cancer MKN-45 cells,and the effects were compared with the effects of SHR-A1403 mAb,the free toxin,or their combination. In the MKN-45 xenograft model,SHR-A1403 was administered at a single dose of 10 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric adenocarcinoma | MKN45 cells | CVCL_0434 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.60% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
SHR-A1403 was evaluated in xenograft mice bearing cancer cells with high c-Met expression,including hepatic cancer HCCLM3,lung cancer NCI-H1993,and gastric cancer MKN-45 cells,and the effects were compared with the effects of SHR-A1403 mAb,the free toxin,or their combination. In the NCI-H1993 xenograft model,SHR-A1403 was administered at a single dose of 10 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1993 cells | CVCL_1512 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.80% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
Effects of SHR-A1403 were further determined in vivo by assessing the growth of HCC827 and HA1 xenograft tumors. Tumor-bearing mice,established by s.c. inoculation of HCC827 and HA1 cells,were randomized into vehicle,AZD9291 (3 mg/kg single dose,i.g.) and SHR-A1403 (10 mg/kg single dose,i.v.) treatment groups when average tumor volumes reached approximately 100-200 mm3.
Click to Show/Hide
|
||||
| In Vivo Model | Non-small cell lung cancer CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells | CVCL_2063 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.80% (Day 21) | High MET expression (MET+++) | ||
| Method Description |
SHR-A1403 was evaluated in xenograft mice bearing cancer cells with high c-Met expression,including hepatic cancer HCCLM3,lung cancer NCI-H1993,and gastric cancer MKN-45 cells,and the effects were compared with the effects of SHR-A1403 mAb,the free toxin,or their combination. In the HCCLM3 xenograft model,SHR-A1403 was administered at a single dose of 10 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Hepatic cancer CDX model | ||||
| In Vitro Model | Adult hepatocellular carcinoma | HCCLM3 cells | CVCL_6832 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.20 ng/mL
|
High MET expression (MET+++; IHC 3+) | ||
| Method Description |
The effects of SHR-A1403 on the proliferation of various types of human cancer cells were evaluated and compared with the effects of SHR-A1403 mAb and the free toxin SHR152852.
|
||||
| In Vitro Model | Adult hepatocellular carcinoma | HCCLM3 cells | CVCL_6832 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.80 ng/mL
|
Negative MET expression (MET-) | ||
| Method Description |
The effects of SHR-A1403 on the proliferation of various types of human cancer cells were evaluated and compared with the effects of SHR-A1403 mAb and the free toxin SHR152852.
|
||||
| In Vitro Model | Gastric adenocarcinoma | MKN45 cells | CVCL_0434 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.80 ng/mL
|
Moderate MET expression (MET++) | ||
| Method Description |
To establish the EGFR inhibitor-resistant NSCLC cells,HCC827 cells were grown initially in medium containing 10 nmol/L gefitinib or afatinib. To exam the ability of the ADC,SHR-A1403,to overcome AZD9291 resistance. Human tumor xenografts were established by s.c. inoculation of nude mice with HCC827,HA1 or HG3 cells. Tumor-bearing mice were randomized into groups and treated with vehicle,AZD9291 intragastric administration (i.g.) or SHR-A1403 intravenous injection (i.v.) when average tumor volume reached approximately 100-200 mm3. Resistance ratio = IC50 (resistant cells)/IC50 (HCC827).
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells (Afatinib resistant; HA1) | CVCL_2063 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
16.30 ng/mL
|
High MET expression (MET+++) | ||
| Method Description |
The effects of SHR-A1403 on the proliferation of various types of human cancer cells were evaluated and compared with the effects of SHR-A1403 mAb and the free toxin SHR152852.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1993 cells | CVCL_1512 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.80 ng/mL
|
High MET expression (MET+++) | ||
| Method Description |
To establish the EGFR inhibitor-resistant NSCLC cells,HCC827 cells were grown initially in medium containing 10 nmol/L gefitinib or afatinib. To exam the ability of the ADC,SHR-A1403,to overcome AZD9291 resistance. Human tumor xenografts were established by s.c. inoculation of nude mice with HCC827,HA1 or HG3 cells. Tumor-bearing mice were randomized into groups and treated with vehicle,AZD9291 intragastric administration (i.g.) or SHR-A1403 intravenous injection (i.v.) when average tumor volume reached approximately 100-200 mm3. Resistance ratio = IC50 (resistant cells)/IC50 (HCC827).
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells (Gefitinib resistant) | CVCL_2063 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
26.60 ng/mL
|
Negative MET expression (MET-) | ||
| Method Description |
To establish the EGFR inhibitor-resistant NSCLC cells,HCC827 cells were grown initially in medium containing 10 nmol/L gefitinib or afatinib. To exam the ability of the ADC,SHR-A1403,to overcome AZD9291 resistance. Human tumor xenografts were established by s.c. inoculation of nude mice with HCC827,HA1 or HG3 cells. Tumor-bearing mice were randomized into groups and treated with vehicle,AZD9291 intragastric administration (i.g.) or SHR-A1403 intravenous injection (i.v.) when average tumor volume reached approximately 100-200 mm3. Resistance ratio = IC50 (resistant cells)/IC50 (HCC827).
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells (Gefitinib resistant) | CVCL_2063 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
46.70 ng/mL
|
Moderate MET expression (MET++) | ||
| Method Description |
The effects of SHR-A1403 on the proliferation of various types of human cancer cells were evaluated and compared with the effects of SHR-A1403 mAb and the free toxin SHR152852.
|
||||
| In Vitro Model | Lung papillary adenocarcinoma | NCI-H441 cells | CVCL_1561 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
53.90 ng/mL
|
High MET expression (MET+++; IHC 3+) | ||
| Method Description |
The effects of SHR-A1403 on the proliferation of various types of human cancer cells were evaluated and compared with the effects of SHR-A1403 mAb and the free toxin SHR152852.
|
||||
| In Vitro Model | Invasive breast carcinoma | Hs 578T cells | CVCL_0332 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
78.20 ng/mL
|
High MET expression (MET+++) | ||
| Method Description |
The effects of SHR-A1403 on the proliferation of various types of human cancer cells were evaluated and compared with the effects of SHR-A1403 mAb and the free toxin SHR152852.
|
||||
| In Vitro Model | Prostate carcinoma | PC-3 cells | CVCL_0035 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
99.40 ng/mL
|
Moderate MET expression (MET++) | ||
| Method Description |
To establish the EGFR inhibitor-resistant NSCLC cells,HCC827 cells were grown initially in medium containing 10 nmol/L gefitinib or afatinib. To exam the ability of the ADC,SHR-A1403,to overcome AZD9291 resistance. Human tumor xenografts were established by s.c. inoculation of nude mice with HCC827,HA1 or HG3 cells. Tumor-bearing mice were randomized into groups and treated with vehicle,AZD9291 intragastric administration (i.g.) or SHR-A1403 intravenous injection (i.v.) when average tumor volume reached approximately 100-200 mm3. Resistance ratio = IC50 (resistant cells)/IC50 (HCC827).
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells (Gefitinib resistant) | CVCL_2063 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
130.80 ng/mL
|
High MET expression (MET+++) | ||
| Method Description |
To establish the EGFR inhibitor-resistant NSCLC cells,HCC827 cells were grown initially in medium containing 10 nmol/L gefitinib or afatinib. To exam the ability of the ADC,SHR-A1403,to overcome AZD9291 resistance. Human tumor xenografts were established by s.c. inoculation of nude mice with HCC827,HA1 or HG3 cells. Tumor-bearing mice were randomized into groups and treated with vehicle,AZD9291 intragastric administration (i.g.) or SHR-A1403 intravenous injection (i.v.) when average tumor volume reached approximately 100-200 mm3. Resistance ratio = IC50 (resistant cells)/IC50 (HCC827).
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells (Gefitinib resistant) | CVCL_2063 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
214.80 ng/mL
|
Moderate MET expression (MET++) | ||
| Method Description |
The effects of SHR-A1403 on the proliferation of various types of human cancer cells were evaluated and compared with the effects of SHR-A1403 mAb and the free toxin SHR152852.
|
||||
| In Vitro Model | Clear cell renal cell carcinoma | Caki-1 cells | CVCL_0234 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
918.90 ng/mL
|
Moderate MET expression (MET++) | ||
| Method Description |
To establish the EGFR inhibitor-resistant NSCLC cells,HCC827 cells were grown initially in medium containing 10 nmol/L gefitinib or afatinib. To exam the ability of the ADC,SHR-A1403,to overcome AZD9291 resistance. Human tumor xenografts were established by s.c. inoculation of nude mice with HCC827,HA1 or HG3 cells. Tumor-bearing mice were randomized into groups and treated with vehicle,AZD9291 intragastric administration (i.g.) or SHR-A1403 intravenous injection (i.v.) when average tumor volume reached approximately 100-200 mm3. Resistance ratio = IC50 (resistant cells)/IC50 (HCC827).
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells | CVCL_2063 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1987.50 ng/mL
|
High MET expression (MET+++; IHC 3+) | ||
| Method Description |
The effects of SHR-A1403 on the proliferation of various types of human cancer cells were evaluated and compared with the effects of SHR-A1403 mAb and the free toxin SHR152852.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 ug/mL | High MET expression (MET+++) | ||
| Method Description |
The effects of SHR-A1403 on the proliferation of various types of human cancer cells were evaluated and compared with the effects of SHR-A1403 mAb and the free toxin SHR152852.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 30.00 ug/mL | Negative MET expression (MET-) | ||
| Method Description |
To establish the EGFR inhibitor-resistant NSCLC cells,HCC827 cells were grown initially in medium containing 10 nmol/L gefitinib or afatinib. To exam the ability of the ADC,SHR-A1403,to overcome AZD9291 resistance. Human tumor xenografts were established by s.c. inoculation of nude mice with HCC827,HA1 or HG3 cells. Tumor-bearing mice were randomized into groups and treated with vehicle,AZD9291 intragastric administration (i.g.) or SHR-A1403 intravenous injection (i.v.) when average tumor volume reached approximately 100-200 mm3. Resistance ratio = IC50 (resistant cells)/IC50 (HCC827).
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | HCC827 cells (Afatinib resistant; HA2) | CVCL_2063 | ||
References
