Payload Information
General Information of This Payload
| Payload ID | PAY0IXEJQ |
|||||
|---|---|---|---|---|---|---|
| Name | AGD-0182 |
|||||
| Synonyms |
AGD-0182; CHEMBL5205301; SCHEMBL17302870; HY-144585; CS-0432631
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
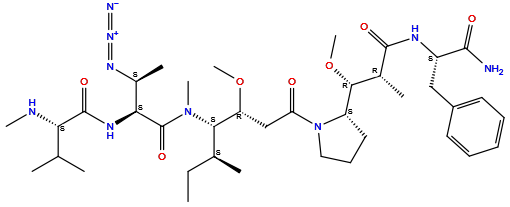
|
|||||
| Formula | C38H63N9O7 |
|||||
| Isosmiles | [H]c1c([H])c([H])c(C([H])([H])[C@@]([H])(C(=O)N([H])[H])N([H])C(=O)[C@]([H])(C([H])([H])[H])[C@@]([H])(OC([H])([H])[H])[C@@]2([H])N(C(=O)C([H])([H])[C@@]([H])(OC([H])([H])[H])[C@@]([H])(N(C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])[C@@]([H])(N=[N+]=[N-])C([H])([H])[H])C([H])([H])[H])[C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C2([H])[H])c([H])c1[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C38H63N9O7/c1-11-23(4)33(46(8)38(52)32(25(6)44-45-40)43-37(51)31(41-7)22(2)3)29(53-9)21-30(48)47-19-15-18-28(47)34(54-10)24(5)36(50)42-27(35(39)49)20-26-16-13-12-14-17-26/h12-14,16-17,22-25,27-29,31-34,41H,11,15,18-21H2,1-10H3,(H2,39,49)(H,42,50)(H,43,51)/t23-,24+,25-,27-,28-,29+,31-,32-,33-,34+/m0/s1
|
|||||
| InChIKey |
TYROHBPOIIUEQF-HFQNRHNCSA-N
|
|||||
| IUPAC Name |
(2S)-N-[(2S,3S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-azido-1-oxobutan-2-yl]-3-methyl-2-(methylamino)butanamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
757.978 |
Polar area |
221.16 |
||
Complexity |
757.4850454 |
xlogp Value |
2.5471 |
|||
Heavy Count |
54 |
Rot Bonds |
32 |
|||
Hbond acc |
9 |
Hbond Donor |
4 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.6 | nM |
Karpas-299 cells/Karpas BVR cells
|
ALK-positive anaplastic large cell lymphoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.8 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[2] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
AGS-62P1 [Phase 1 (Terminated)]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
27.00%
|
Positive FLT3 Expression (FLT3+++/++) | ||
| Method Description |
The in vivo antitumor activity of ASP1235 was evaluated in a FLT3 positive THP-1 xenograft model. THP-1 cells were subcutaneously inoculated in the right frank of mice at 5 x106 cells/head. The vehicle of each drug is shown as follows: ASP1235 (20 mM Histidine/L-Histidine-HCl, 5.5% trehalose dihydrate, 0.01% Polysorbate 20, pH6.0), venetoclax (60% phosal 50PG, 30% PEG400, 10% ethanol), azacitidine (PBS). ASP1235 was intravenously administrated to each mouse once per week. One day before ASP1235 treatment, each mouse received intraperitoneal injection of 20 mg/kg of nonspecific human IgG1 antibody. Venetoclax was orally administered daily at 100 mg/kg. Azacitidine was intravenously injected at 3 mg/kg for five consecutive days in the first week of treatment.
Click to Show/Hide
|
||||
| In Vivo Model | FLT3-expressing THP-1 xenograft model | ||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
35.10%
|
Positive FLT3 Expression (FLT3+++/++) | ||
| Method Description |
The in vivo antitumor activity of ASP1235 was evaluated in a FLT3 positive THP-1 xenograft model. THP-1 cells were subcutaneously inoculated in the right frank of mice at 5 x106 cells/head. The vehicle of each drug is shown as follows: ASP1235 (20 mM Histidine/L-Histidine-HCl, 5.5% trehalose dihydrate, 0.01% Polysorbate 20, pH6.0), venetoclax (60% phosal 50PG, 30% PEG400, 10% ethanol), azacitidine (PBS). ASP1235 was intravenously administrated to each mouse once per week. One day before ASP1235 treatment, each mouse received intraperitoneal injection of 20 mg/kg of nonspecific human IgG1 antibody. Venetoclax was orally administered daily at 100 mg/kg. Azacitidine was intravenously injected at 3 mg/kg for five consecutive days in the first week of treatment.
Click to Show/Hide
|
||||
| In Vivo Model | FLT3-expressing THP-1 xenograft model | ||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
38.00%
|
Positive FLT3 Expression (FLT3+++/++) | ||
| Method Description |
The in vivo antitumor activity of ASP1235 was evaluated in a FLT3 positive THP-1 xenograft model. THP-1 cells were subcutaneously inoculated in the right frank of mice at 5 x106 cells/head. The vehicle of each drug is shown as follows: ASP1235 (20 mM Histidine/L-Histidine-HCl, 5.5% trehalose dihydrate, 0.01% Polysorbate 20, pH6.0), venetoclax (60% phosal 50PG, 30% PEG400, 10% ethanol), azacitidine (PBS). ASP1235 was intravenously administrated to each mouse once per week. One day before ASP1235 treatment, each mouse received intraperitoneal injection of 20 mg/kg of nonspecific human IgG1 antibody. Venetoclax was orally administered daily at 100 mg/kg. Azacitidine was intravenously injected at 3 mg/kg for five consecutive days in the first week of treatment.
Click to Show/Hide
|
||||
| In Vivo Model | FLT3-expressing THP-1 xenograft model | ||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
84.00%
|
Positive FLT3 Expression (FLT3+++/++) | ||
| Method Description |
The in vivo antitumor activity of ASP1235 was evaluated in a FLT3 positive THP-1 xenograft model. THP-1 cells were subcutaneously inoculated in the right frank of mice at 5 x106 cells/head. The vehicle of each drug is shown as follows: ASP1235 (20 mM Histidine/L-Histidine-HCl, 5.5% trehalose dihydrate, 0.01% Polysorbate 20, pH6.0), venetoclax (60% phosal 50PG, 30% PEG400, 10% ethanol), azacitidine (PBS). ASP1235 was intravenously administrated to each mouse once per week. One day before ASP1235 treatment, each mouse received intraperitoneal injection of 20 mg/kg of nonspecific human IgG1 antibody. Venetoclax was orally administered daily at 100 mg/kg. Azacitidine was intravenously injected at 3 mg/kg for five consecutive days in the first week of treatment.
Click to Show/Hide
|
||||
| In Vivo Model | FLT3-expressing THP-1 xenograft model | ||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20-12.00 nM
|
Positive FLT3 Expression (FLT3+++/++) | ||
| Method Description |
The cytotoxic activity of AGS-62P1 was evaluated against a panel of AmL cell lines in vitro.
|
||||
| In Vitro Model | Acute myeloid leukemia | Acute myeloid leukemia cells | Homo sapiens | ||
References
