Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0YFUMC
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Tras-Gly5-EDA-Dox
|
|||||
| Synonyms |
Tras-Gly5-Dox; Tras Gly5 Dox
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.7-3.9
|
|||||
| Structure |
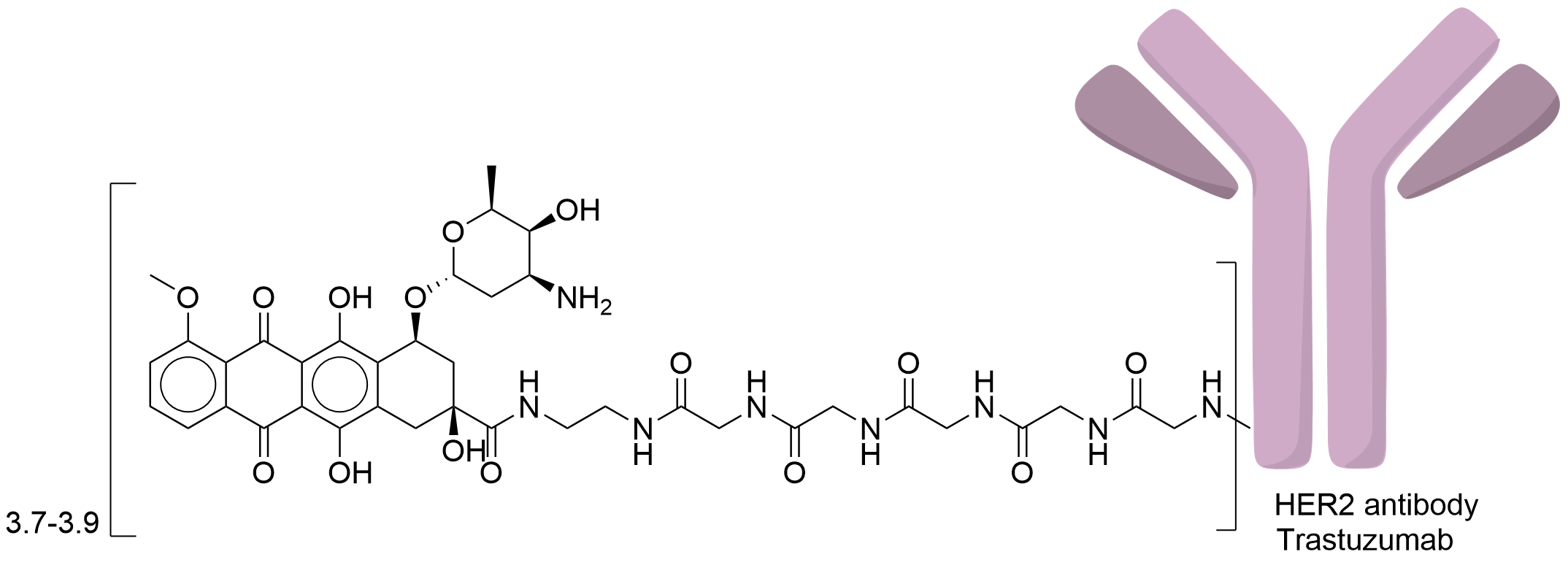
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Doxorubicin
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 2-alpha (TOP2A)
|
Target Info | ||||
| Linker Name |
Gly5-EDA
|
Linker Info | ||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 2.80 ng/mL | High HER2 expression (HER2+++; 694,000 HER2 molecules/cell) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
References
