Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0YANSF
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
BL-B01D1
|
|||||
| Synonyms |
BL B01D1; BL-B01D1; BLB01D1
Click to Show/Hide
|
|||||
| Organization |
Sichuan Baili Pharmaceutical Co., Ltd.; Systimmune, Inc.; Chengdu Bailidote Biological Pharmaceutical Co., Ltd.
|
|||||
| Drug Status |
Phase 3
|
|||||
| Indication |
In total 7 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
8
|
|||||
| Structure |
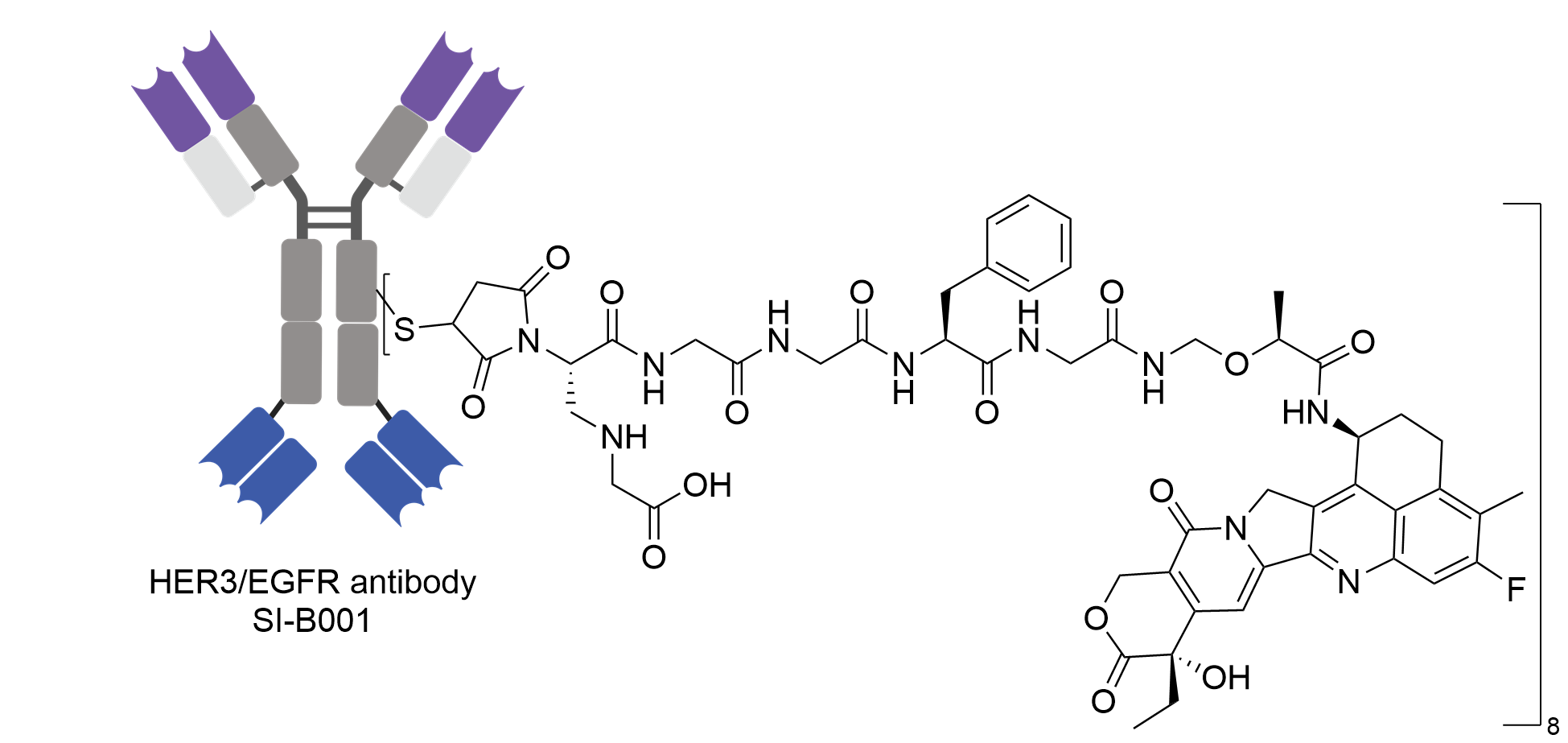
|
|||||
| Antibody Name |
SI-B001
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-3 (HER3); Epidermal growth factor receptor (EGFR)
|
Antigen Info | ||||
| Payload Name |
Camptothecin analogue ED04
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Gly-Mal-Gly-Gly-Phe-Gly
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
brengitecan
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
61.80% (NSCLC EGFR mutation)
40.50% (NSCLC EGFR wildtype) 14.30% (SCLC) 45.80% (NPC) 7.70% (HNSCC) |
|||
| Patients Enrolled |
Patients with locally advanced or metastatic solid tumors.
|
||||
| Administration Dosage |
BL-B01D1 was administered intravenously at doses of 2.50, 3.00 mg/kg D1D8 Q3W and 4.50, 5.00, 6.00 mg/kg D1 Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05194982 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 clinical study to evaluate the safety, tolerability, pharmacokinetic characteristics and preliminary efficacy of BL-B01D1 in patients with locally advanced or metastatic solid tumor. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05785039 | Clinical Status | Phase 2 | ||
| Clinical Description | Phase 2a/2b clinical study to evaluate the safety, tolerability, pharmacokinetics and efficacy of BL-B01D1 for injection in patients with multiple solid tumors such as locally advanced or metastatic urinary system tumors. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05470348 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 clinical study to evaluate the safety, tolerability, pharmacokinetic characteristics and preliminary efficacy of BL-B01D1 in patients with unresectable locally advanced or metastatic breast cancer and other solid tumors. | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05393427 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 clinical study to evaluate the safety, tolerability, pharmacokinetic characteristics and preliminary efficacy of BL-B01D1 in patients with locally advanced or metastatic urological tumors and other solid tumors. | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05262491 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 clinical study to evaluate the safety, tolerability, pharmacokinetic characteristics and preliminary efficacy of BL-B01D1 in patients with locally advanced or metastatic gastrointestinal tumor and other solid tumor. | ||||
References
