Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0XKZBO
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
AZD8205
|
|||||
| Synonyms |
AZD 8205; AZD-8205; AZD8205
Click to Show/Hide
|
|||||
| Organization |
AstraZeneca PLC; BSP Pharmaceuticals SpA
|
|||||
| Drug Status |
Phase 1/2
|
|||||
| Indication |
In total 5 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
8
|
|||||
| Structure |
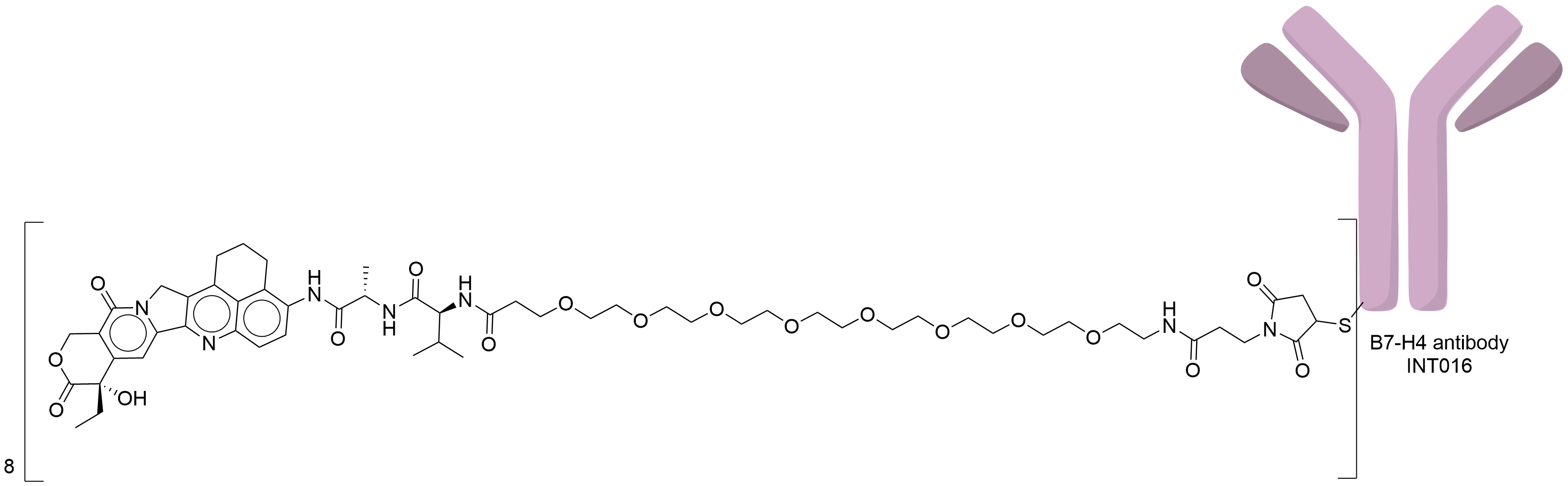
|
|||||
| Antibody Name |
Puxitatug
|
Antibody Info | ||||
| Antigen Name |
V-set domain-containing T-cell activation inhibitor 1 (VTCN1)
|
Antigen Info | ||||
| Payload Name |
AZ0132
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Mal-PEG8-Val-Ala
|
Linker Info | ||||
| Conjugate Type |
Reactive Cysteines
|
|||||
| Combination Type |
samrotecan
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
| Standard Type | Value | Units | Animal Model (No. of PDX) |
|---|---|---|---|
| Objective Response Rate (ORR) |
69
|
%
|
Multiple tumor PDX model
|
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
Patients 18 years old with cholangiocarcinoma, breast, ovarian or endometrial cancers and ECOG PS 0-1.
|
||||
| Administration Dosage |
.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05123482 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1/2a multi-center, open-label master protocol to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary antitumor activity of AZD8205 in participants with advanced or metastatic solid malignancies. | ||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 69.00% | Positive VTCN1 expression (VTCN1+++/++) | ||
| Method Description |
In the study of 26 PDX tumors,single administration of 3.5 mg/kg AZD8205 to determine the ORR,according to modified RECIST criteria,which correlated with homologous recombination repair (HRR) deficiency (HRD) and elevated levels of B7-H4 in HRR-proficient models.
|
||||
| In Vivo Model | Multiple tumor PDX model | ||||
References
