Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0WRMGX
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Anti-TNF ADC 134
|
|||||
| Synonyms |
Anti-TNF-ADC-134
Click to Show/Hide
|
|||||
| Organization |
AbbVie, Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
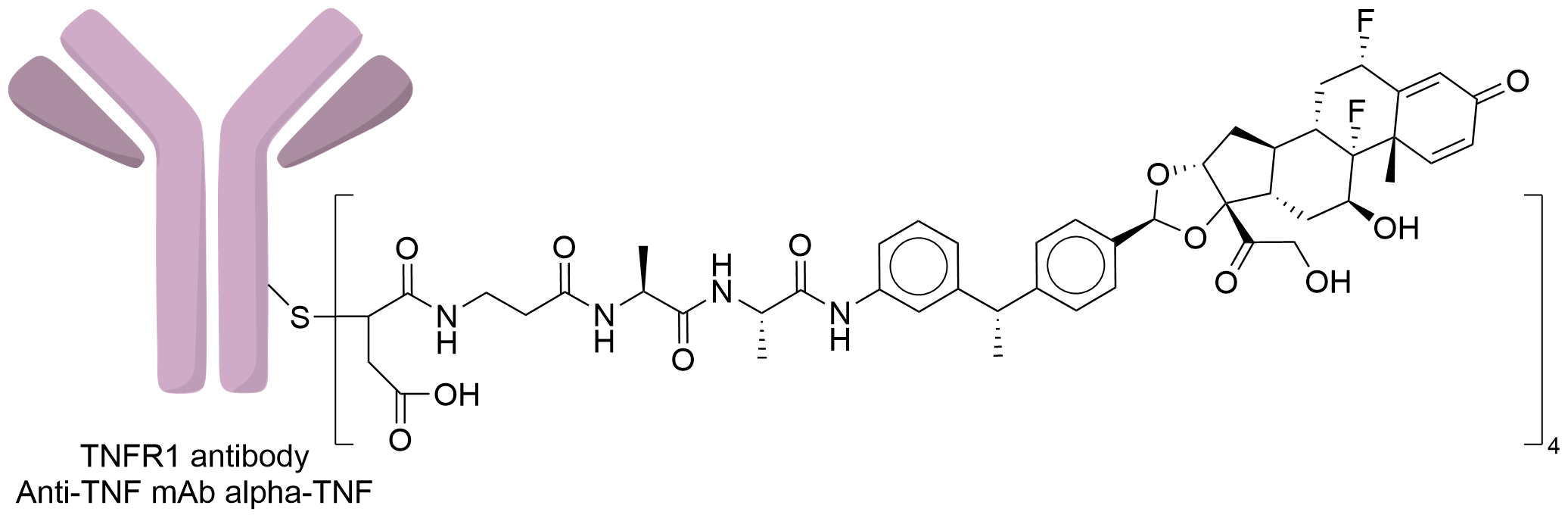
|
|||||
| Antibody Name |
Anti-TNF mAb alpha-TNF
|
Antibody Info | ||||
| Antigen Name |
Tumor necrosis factor receptor superfamily member 1A (TNFRSF1A)
|
Antigen Info | ||||
| Payload Name |
GRM cpd 64
|
Payload Info | ||||
| Therapeutic Target |
Glucocorticoid receptor (NR3C1)
|
Target Info | ||||
| Linker Name |
MP-Ala-Ala
|
Linker Info | ||||
| Conjugate Type |
The free sulfhydryl group obtained by the reduction of interchain cysteine is site-specific conjugated with maleimide, michael addition reaction, followed by incubation at basic ph to hydrolyze-open the maleimide ring.
|
|||||
General Information of The Activity Data Related to This ADC
Obtained from the Model Organism Data
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | P1NP inhibition | 25.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess P1NP level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | P1NP inhibition | 58.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess P1NP level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Ear swelling inhibition | 72.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization.
|
||||
| In Vivo Model | Fluorescein isothiocyanate (FITC)-induced CHS model | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Ear swelling inhibition | 94.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization.
|
||||
| In Vivo Model | Fluorescein isothiocyanate (FITC)-induced CHS model | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Corticosterone inhibition | 36.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess corticosterone level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Corticosterone inhibition | 74.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess corticosterone level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.80 ng/mL | Positive TNF expression (TNF+++/++) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells (TNF expression) | CVCL_0004 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 10.70 ug/mL | Negative TNF expression (TNF-) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells | CVCL_0004 | ||
References
