Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0WCYUM
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Lupartumab amadotin
|
|||||
| Synonyms |
Anti-C4.4a antibody-drug conjugates; Anti-LY6-PLAUR domain containing 3 antibody-drug conjugates; Anti-LYPD3 antibody-drug conjugates; BAY 1129980; BAY-1129980; BAY1129980; LYPD3 protein-directed antibody-drug conjugates; Lupartumab amadotin
Click to Show/Hide
|
|||||
| Organization |
Bayer AG
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
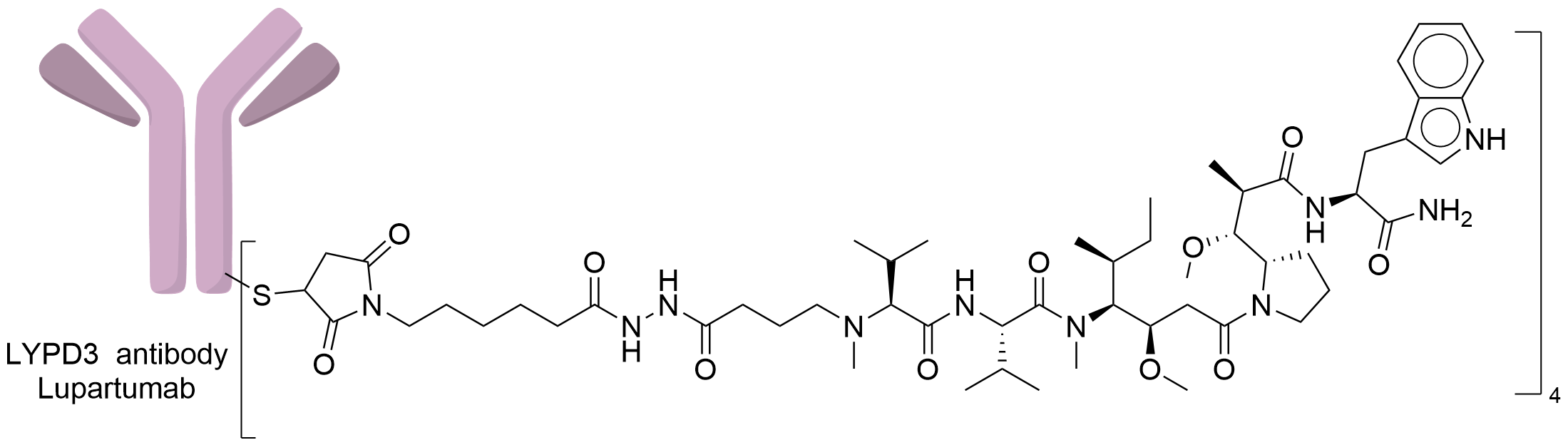
|
|||||
| Antibody Name |
Lupartumab
|
Antibody Info | ||||
| Antigen Name |
Ly6/PLAUR domain-containing protein 3 (LYPD3)
|
Antigen Info | ||||
| Payload Name |
Auristatin W
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-hydrazide linker
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
amadotin
|
|||||
| Puchem SID | ||||||
| TTD ID | ||||||
