Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0UXODO
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
EphA2-targeted mAb ADC B 17 (DAR8)
|
|||||
| Synonyms |
EphA2-targeted-mAb-ADC-B-17-(DAR8)
Click to Show/Hide
|
|||||
| Organization |
Seagen Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
8
|
|||||
| Structure |
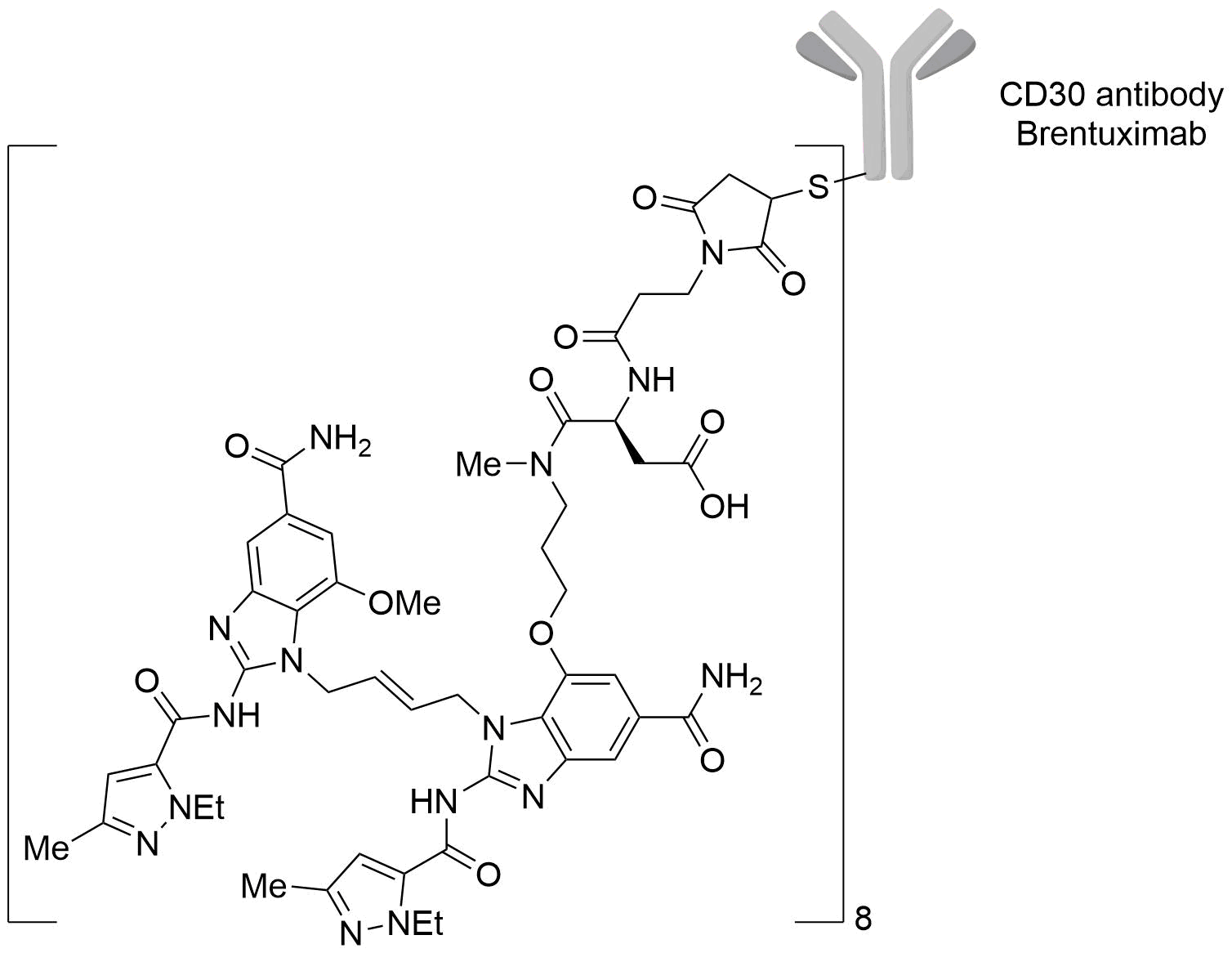
|
|||||
| Antibody Name |
Brentuximab
|
Antibody Info | ||||
| Antigen Name |
Tumor necrosis factor receptor superfamily member 8 (TNFRSF8)
|
Antigen Info | ||||
| Payload Name |
Undisclosed
|
|||||
| Linker Name |
EphA2-targeted mAb ADC B 17 (DAR8) linker
|
|||||
General Information of The Activity Data Related to This ADC
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.20 ng/mL | Positive EPHA2 expression (EPHA2+++/++); Positive CD30 expression (CD30+++/++) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the L540cy cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Hodgkin's disease | L540cy cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 1.00 ng/mL | Positive EPHA2 expression (EPHA2+++/++); Positive CD30 expression (CD30+++/++) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the Karpas-299 cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 2.00 ng/mL | Positive EPHA2 expression (EPHA2+++/++); Negative CD30 expression (CD30-) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the MOLM-13 cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | MOLM-13 cells | CVCL_2119 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 1000 ng/mL | Positive EPHA2 expression (EPHA2+++/++); Negative CD30 expression (CD30-) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the 786-O cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 1000 ng/mL | Positive EPHA2 expression (EPHA2+++/++); Negative CD30 expression (CD30-) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the A2058 cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Melanoma | A2058 cells | CVCL_1059 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 1000 ng/mL | Positive EPHA2 expression (EPHA2+++/++); Negative CD30 expression (CD30-) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the BxPC3 cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 1000 ng/mL | Positive EPHA2 expression (EPHA2+++/++); Negative CD30 expression (CD30-) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the Ls174T cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Colon adenocarcinoma | LS174T cells | CVCL_1384 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 1000 ng/mL | Positive EPHA2 expression (EPHA2+++/++); Negative CD30 expression (CD30-) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the MDA-MB-231 cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 1000 ng/mL | Positive EPHA2 expression (EPHA2+++/++); Negative CD30 expression (CD30-) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the SU-DHL-4 cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | SU-DHL-4 cells | CVCL_0539 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 4.00 pg/mL | Positive EPHA2 expression (EPHA2+++/++); Positive CD30 expression (CD30+++/++) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the DEL cells (Brentuximab vedotin resistance). Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Anaplastic large cell lymphoma | DEL cells (Brentuximab vedotin resistant) | CVCL_1170 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 4.00 pg/mL | Positive EPHA2 expression (EPHA2+++/++); Positive CD30 expression (CD30+++/++) | ||
| Method Description |
Potency of compounds and ADCs was evaluated using the DEL cells. Cells were plated in a 96-well flat bottom tissue culture-treated clear polystyrene plate at ~100,000 cells per well in 200 uL with the indicated concentration of the compound or ADC.
|
||||
| In Vitro Model | Anaplastic large cell lymphoma | DEL cells | CVCL_1170 | ||
