Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0SXFSY
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Inotuzumab ozogamicin
|
|||||
| Brand Name |
Besponsa
|
|||||
| Synonyms |
CMC-544;PF-05208773;PF-5208773;WAY-207294;INO
Click to Show/Hide
|
|||||
| Organization |
Pfizer Inc.; Wyeth Pharmaceuticals LLC
|
|||||
| Drug Status |
Approved (FDA): Aug, 2017
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
6
|
|||||
| Structure |
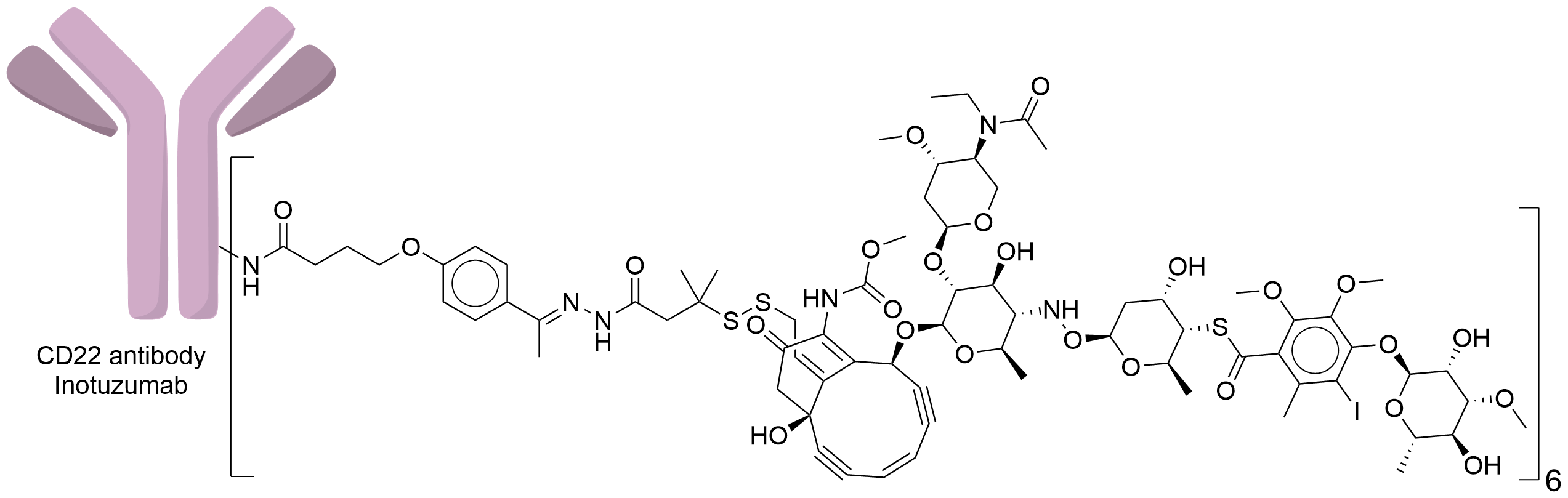
|
|||||
| Antibody Name |
Inotuzumab
|
Antibody Info | ||||
| Antigen Name |
B-cell receptor CD22 (CD22)
|
Antigen Info | ||||
| Payload Name |
N-acetyl-gamma-calicheamicin
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
AcButDMH
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
Ozogamicin
|
|||||
| Absorption |
Inotuzumab ozogamicin is intended to be administered in cycles that each run for 3 to 4 weeks. The steady state exposure of the drug is reached by Cycle 4. The mean (SD) maximum serum concentration (Cmax) of inotuzumab ozogamicin was 308 ng/mL with patients receving the recommended dose of 1.8 mg/m2/cycle.
|
|||||
| Distribution |
The total volume of distribution of inotuzumab ozogamicin is approximately 12L. The binding of the N-acetyl-gamma-calicheamicin dimethylhydrazide to human plasma proteins to be approximately 97%.
|
|||||
| Metabolism |
N-acetyl-gamma-calicheamicin dimethylhydrazide primarily undergoes nonenzymatic reduction in vitro. The metabolism of N-acetyl-gamma-calicheamicin dimethylhydrazide in human serum is not clearly understood. The antibody portion of the drug is thought to undergo proteolytic degradation into amino acids then recycled into other proteins. The elimination half life at the end of Cycle 4 of administration is approximately 12.3 days in a 2-compartment model.
|
|||||
| Elimination |
The drug is disposited in the body after administration. The clearance of inotuzumab ozogamicin at steady state is 0.0333 L/h.
|
|||||
| Toxicity |
Inotuzumab ozogamicin has the potential to impair reproductive function and fertility in men and women. Hepatotoxicity, including hepatic veno-occlusive disease (VOD) (also known as sinusoidal obstruction syndrome and increased risk of post-hematopoietic stem cell transplant (HSCT) non-relapse mortality.
|
|||||
| Special Approval(s) |
Breakthrough therapy(FDA); Orphan drug(FDA); Orphan drug(EMA)
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
| DRESIS ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) | 60.00% (DL2a), 33.00% (DL1a), 25.00% (DL1) | Positive CD22 expression (CD22+++/++) | ||
| Patients Enrolled |
Relapsed/refractory CD22 positive B-cell non-Hodgkin lymphomas.
|
||||
| Administration Dosage |
InO 0.80 mg/m2 on d1 and TEM 15 mg d1,8,15,22 q28d (of the four patients included in dose level (DL) 1 ); InO 0.80 mg/m2 on d1 and TEM 10 mg d1,8,15,22 q28d (DL-1) w.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01535989 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of inotuzumab ozogamicin (CMC-544) in combination with temsirolimus (CCI-779) in patients with relapsed or refractory CD22-positive B-cell non Hodgkin's lymphomas. | ||||
| Primary Endpoint |
Anti-tumor activity was observed in three of the four dose levels tested (PR 60.00% in DL2a, 33.00% in DL1a, and 25.00% in DL1).
|
||||
| Other Endpoint |
Patients received a total of 47 cycles of study treatment with a median of two cycles (range 16). Of the four patients included in dose level (DL) 1 (InO 0.80 mg/m2 on d1 and TEM 15 mg d1, 8, 15, 22 q28d), two patients experienced DLTs (grade 4 thrombocytopenia and inability to receive 75% planned dose of TEM in one patient and grade 3 hypertriglyceridemia in another) and DL-1 (InO 0.80 mg/m2 on d1 and TEM 10 mg d1, 8, 15, 22 q28d) was evaluated again with the occurrence of DLTs in the first treated patient (grade 3 general physical health deterioration and inability to receive at least 3/4 doses of TEM).
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 74.00% | Positive CD22 expression (CD22+++/++) | ||
| Patients Enrolled |
Patients were eligible if they were 21 years of age at the time of InO administration and had received a minimum of one dose of InO.
|
||||
| Administration Dosage |
One cycle consisted of three doses: 0.8 mg/m2 on week 1 followed by 0.5 mg/m2 on weeks 2 and 3.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02981628 | Clinical Status | Phase 2 | ||
| Clinical Description | Inotuzumab ozogamicin in treating younger patients with B-lymphoblastic lymphoma or relapsed or refractory CD22 positive B acute lymphoblastic leukemia. | ||||
| Primary Endpoint |
Complete responses were reported in 28 of 42 (66.67%) patients with overt relapse (M2/M3 marrow): CR in 15 (35.71%) and CRi in 13 (30.95%).
|
||||
| Other Endpoint |
Three patients had a partial response (7.14%) and eight had no response (19.05%).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 39.24% (all 79 patients), 40.82% (for the 49 patients treated at the MTD), 68.00% (for patients with FL), 15.00% (for patients with DLBCL) | Positive CD22 expression (CD22+++/++) | ||
| Patients Enrolled |
Relapsed or refractory CD22+ B-cell non-Hodgkin's lymphoma (NHL).
|
||||
| Administration Dosage |
Once every 3 or 4 weeks at doses ranging from 0.40 to 2.40 mg/m2.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00073749 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of Cmc-544 administered as a single agent in subjects with B-cell non- Hodgkin's lymphoma. | ||||
| Primary Endpoint |
Among all 79 patients across all doses, the ORR at the end of treatment was 39.24%. For the 49 patients treated at the MTD, the ORR was 40.82% (95% CI, 27.00% to 56.00%). For patients with FL, ORR was 68.00% (95% CI, 45.00% to 86.00%); for patients with DLBCL, ORR was 15.00% (95% CI, 4.00% to 35.00%). For the 43 patients enrolled onto the expanded MTD cohort, duration of response ranged from 63 to 644 days for patients with FL (n=18) and from 34 to 685 days for patients with DLBCL (n=25). Median PFS was 317 days (95% CI, 112 to 575 days) for patients with FL and 49 days (95% CI, 28 to 105 days) for patients with DLBCL. Median OS was 193 days (95% CI, 103 to 362 days) for patients with DLBCL.
Click to Show/Hide
|
||||
| Other Endpoint |
The MTD was declared to be 1.80 mg/m2. DLTs also occurred at 1.34 and 1.80 mg/m2 but did not meet criteria for escalation stop.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 80.00% (In the ITT population), 88.00% (In the eight evaluable patients who received two or more cycles of study treatment and had one or more post-baseline tumor assessment) | Positive CD22 expression (CD22+++/++) | ||
| Patients Enrolled |
Relapsed or refractory B-cell non-Hodgkin lymphoma.
|
||||
| Administration Dosage |
A fixed standard dose of rituximab 375 mg/m2 was administered i.v. on day 1 of a 28-day (2 days) cycle followed by inotuzumab ozogamicin 1.80 mg/m2 i.v. on day 2 of the cycle.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00724971 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of Cmc-544 administered in combination with rituximab in subjects with B-cell non-Hodgkin's lymphoma. | ||||
| Primary Endpoint |
Os at 1 year (52 weeks) was 100.00%, as no deaths were observed during the study. In the ITT population, the ORR was 80.00% (95% CI, 44.00-98.00%). In the eight evaluable patients who received two or more cycles of study treatment and had one or more post-baseline tumor assessment, ORR was 88.00% (95% CI, 47.00-99.00%). The duration of response ranged from 346 to 540 days. In the ITT population, the best overall responses (from the start of treat-ment until PD) were CR in six patients. In the evaluable population, CR was achieved in six patients and CRu and SD were achieved in one patient each. The PFS rate at 1 year was 89.00% (95% CI, 43-98%) in the ITT population and 88.00% (95% CI, 39-98%) in the evaluable population.
Click to Show/Hide
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 87.00% (FL), 74.00% (DLBCL), 20.00% (andrefractory disease) | Positive CD22 expression (CD22+++/++) | ||
| Patients Enrolled |
Relapsed follicular lymphoma (FL), relapsed diffuse large B-cell lymphoma (DLBCL), or refractory aggressive NHL (eligible subtypes: DLBCL, transformed FL, follicular grade 3b, or mantle cells). Refractory was defined as disease progression less than 6 months from the start of the most recent rituximab-containing treatment.
|
||||
| Administration Dosage |
Five patients received INO 0.80 mg/m2, three received INO 1.30 mg/m2, and seven received INO 1.80 mg/m2 in combination with rituximab at 375 mg/m2 once every 4 weeksm2.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00299494 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study Of Cmc-544 administered in combination with rituximab in subjects with follicular or diffuse large B-cell non-Hodgkin's lymphoma. | ||||
| Primary Endpoint |
At MTD treatment, objective response rate (ORR) was 87.00%, 74.00%, and 20.00% for patients with FL, DLBCL, andrefractory disease, respectively. Confirmed complete response (CR) and unconfirmed CR were achieved in 62.00% of patients with FL and 50.00% of patients with relapsed DLBCL. Median duration of response was 17.70 months for relapsed DLBCL, 6.10 months for refractory aggressive NHL.
Click to Show/Hide
|
||||
| Other Endpoint |
For FL group, one-year PFS rate was 87.00%; two-year PFS rate was 68.00%. For relapsed FL group, one-year OS rate was 97.00%; two-year OS rate was 90.00%. For relapsed DLBCL group, one-year PFS rate was 55.00%, one-year OS rate was 80.00%; two-year PFS rate was 42.00%, two-year OS rate was 69.00%, median PFS was 17.10 months. In total, 36.00% of patients with refractory disease were alive at 2 years (median OS, 8.80 months).
Click to Show/Hide
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 100.00% (In the 1.3 mg m2 cohort), 80.00% (In the 1.8 mg m2 cohort) | Positive CD22 expression (CD22+++/++) | ||
| Patients Enrolled |
Relapsed or refractory CD22-positive B-NHL without major organ dysfunction.
|
||||
| Administration Dosage |
1.30 mg/m2 administered IV once every 28 days, and dose escalation was performed up to the MTD of 1.80 mg/m2 administered IV once every 28 days.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00717925 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of CMC-544 administered as a single agent in subjects with B-cell non-hodgkin's lymphoma. | ||||
| Primary Endpoint |
Antitumor activity was observed at both dose levels. In the 1.30 mg/m2 cohort, two out of three patients had CR, and one patient had CRu for an ORR of 100.00% (95% CI, 29.00-100.00%). In the 1.80 mg/m2 cohort, one out of 10 patients had CR, three patients had CRu, and four patients had PR for an ORR of 80.00% (95% CI, 44.00-98.00%).
Click to Show/Hide
|
||||
| Experiment 7 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Complete Remission (CR) | 73.80% | Positive CD22 expression (CD22+++/++) | ||
| Patients Enrolled |
Eligible patients were 18 years old, had a diagnosis of R/R CD22 positive BCP ALL.
|
||||
| Administration Dosage |
307 received 1 or more doses of the study drug (164 in the InO arm and 143 in the SoC arm).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01564784 | Clinical Status | Phase 3 | ||
| Clinical Description | A study of inotuzumab ozogamicin versus investigator's choice of chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia. | ||||
| Primary Endpoint |
The complete remission (CR)/complete remission with incomplete hematologic recovery (CRi) rate was higher with InO versus SoC, with consistent CR/CRi rates across patient subgroups. The median overall survival (OS) was 7.7 months with InO and 6.2 months with SoC, with 2 year OS rates of 22.80% and 10.00%, respectively.
|
||||
| Other Endpoint |
More InO arm patients proceeded directly to HSCT after achieving CR/CRi before any followup induction therapy (39.60%vs10.50%).
|
||||
| Experiment 8 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Complete Remission (CR) |
18.00%
|
|||
| Patients Enrolled |
Refractory and relapsed acute lymphocytic leukemia (ALL).
|
||||
| Administration Dosage |
1.80 mg/m2 inotuzumab ozogamicin intravenously over 1 h every 34 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01134575 | Clinical Status | Phase 2 | ||
| Clinical Description | Treatment of relapsed or refractory acute lymphoblastic leukemia (ALL) with CMC-544 (Inotuzumab Ozogamycin), with or without later addition of rituximab. | ||||
| Primary Endpoint |
Median overall survival was 5.10 months (95% CI 3.80-6.40). Median survival for the 28 responders was 7.90 months (95% CI 5.30-10.50). Among the nine patients with complete response, the estimated survival at 12 months was 78.00%.
|
||||
| Other Endpoint |
Among the 19 patients with marrow responses but no platelet or incomplete blood cell count recovery, the median survival was 6.70 months (95% CI 3.90-9.50), and among the remaining 21 patients it was 2.40 months (1.70-3.90).
|
||||
| Experiment 9 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Complete Remission (CR) |
39.60%
|
|||
| Patients Enrolled |
R/R CD22-positive B-acute lymphocytic leukemia(ALL).
|
||||
| Administration Dosage |
0.80 mg/m2 intravenously on day 1 and 0.50 mg/m2 on days 8 and 15 of a 28-day cycle.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02981628 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase 2 study of inotuzumab ozogamicin (NSC# 772518) in children and young adults with relapsed or refractory CD22+ B-acute lymphoblastic leukemia (B-ALL). | ||||
| Primary Endpoint |
Cr=39.60%, CRi=18.80% in cycle 1; CR and CRi=62.50% in cycle 2.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 54.00% (Day 12) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Inotuzumab ozogamicin induces efficient tumor cell killing in CDX model of a Ramos BCL with CD22+, administered intraperitoneally as 3 doses, 1 every 4 days at 10 ug/kg.
|
||||
| In Vivo Model | Ramos BCL cell line xenograft model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.60% (Day 24) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Inotuzumab ozogamicin induces efficient tumor cell killing in CDX model of a small RL BCL with CD22+, administered as 3 doses, 1 every 4 days via either the intraperitoneal or intravenous route at 20 ug/kg.
|
||||
| In Vivo Model | RL BCL cell line xenograft model | ||||
| In Vitro Model | B-cell lymphoma | B-cell lymphoma cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 68.20% (Day 14) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Inotuzumab ozogamicin induces efficient tumor cell killing in CDX model of a developing Ramos BCL with CD22+, administered intraperitoneally as 3 doses, 1 every 4 days at 160 ug/kg.
|
||||
| In Vivo Model | Ramos BCL cell line xenograft model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.80% (Day 14) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Inotuzumab ozogamicin induces efficient tumor cell killing in CDX model of large Ramos BCL ((almost 10% of the body weight) with CD22+, administered intraperitoneally as 3 doses, 1 every 4 days at 160 ug/kg.
|
||||
| In Vivo Model | Large ramos BCL cell line xenograft model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.90% (Day 45) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
CMC-544 induces efficient tumor cell killing in cell line-derived models B-ALL of cells with CD22+, dosed weekly at 10 ug/kg ip Q4D3.
|
||||
| In Vivo Model | REH B-ALL cell line xenograft model | ||||
| In Vitro Model | B acute lymphoblastic leukemia | Reh cells | CVCL_1650 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.10% (Day 45) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
CMC-544 induces efficient tumor cell killing in cell line-derived models B-ALL of cells with CD22+, dosed weekly at 40 ug/kg ip Q4D3.
|
||||
| In Vivo Model | REH B-ALL cell line xenograft model | ||||
| In Vitro Model | B acute lymphoblastic leukemia | Reh cells | CVCL_1650 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 79.10% (Day 12) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Inotuzumab ozogamicin induces efficient tumor cell killing in CDX model of a Ramos BCL with CD22+, administered intraperitoneally as 3 doses, 1 every 4 days at 40 ug/kg.
|
||||
| In Vivo Model | Ramos BCL cell line xenograft model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.60% (Day 45) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
CMC-544 induces efficient tumor cell killing in cell line-derived models B-ALL of cells with CD22+, dosed weekly at 160 ug/kg ip Q4D3.
|
||||
| In Vivo Model | REH B-ALL cell line xenograft model | ||||
| In Vitro Model | B acute lymphoblastic leukemia | Reh cells | CVCL_1650 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.40% (Day 9) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Inotuzumab ozogamicin induces efficient tumor cell killing in CDX models of an s.c. B-cell lymphoma, dosed at 160 ug/kg, i.p., q4d3 to scid mice with s.c. Ramos B-cell lymphoma xenografts.
|
||||
| In Vivo Model | Ramos CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.20% (Day 30) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
CMC-544 induces efficient tumor cell killing in cell line-derived models B-cell lymphoma cell line with CD22+, dosed weekly at 16 ug/kg ip Q4D3.
|
||||
| In Vivo Model | Ramos BCL cell line xenograft model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.40% (Day 24) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Inotuzumab ozogamicin induces efficient tumor cell killing in CDX model of a small RL BCL with CD22+, administered as 3 doses, 1 every 4 days via either the intraperitoneal or intravenous route at 80 ug/kg.
|
||||
| In Vivo Model | RL BCL cell line xenograft model | ||||
| In Vitro Model | B-cell lymphoma | B-cell lymphoma cells | Homo sapiens | ||
| Experiment 12 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.20% (Day 12) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Inotuzumab ozogamicin induces efficient tumor cell killing in CDX model of a Ramos BCL with CD22+, administered intraperitoneally as 3 doses, 1 every 4 days at 160 ug/kg.
|
||||
| In Vivo Model | Ramos BCL cell line xenograft model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.80% (Day 35) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
CMC-544 induces efficient tumor cell killing in cell line-derived models B-cell lymphoma cell line with CD22+, dosed at ip 80 ug/kg Q4Dx3.
|
||||
| In Vivo Model | Ramos BCL cell line xenograft model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.70% (Day 30) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
CMC-544 and rituximab combination induces efficient tumor cell killing in cell line-derived models B-cell lymphoma cell line with CD22+, dosed ip CMC-544 (16 ug/kg Q4D3) and Rituximab (2 mg/kg Q4D3).
|
||||
| In Vivo Model | Ramos BCL cell line xenograft model | ||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.30% (Day 35) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
CMC-544 induces efficient tumor cell killing in cell line-derived models B-cell lymphoma cell line with lower CD22 expression, dosed at ip 160 ug/kg Q4Dx3.
|
||||
| In Vivo Model | RL BCL cell line xenograft model | ||||
| In Vitro Model | B-cell lymphoma | B-cell lymphoma cells | Homo sapiens | ||
| Experiment 16 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.80% (Day 24) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Inotuzumab ozogamicin induces efficient tumor cell killing in CDX model of a small RL BCL with CD22+, administered as 3 doses, 1 every 4 days via either the intraperitoneal or intravenous route at 320 ug/kg.
|
||||
| In Vivo Model | RL BCL cell line xenograft model | ||||
| In Vitro Model | B-cell lymphoma | B-cell lymphoma cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 pM
|
|||
| Method Description |
The inhibitory activity of CMC-544 combining with rituximab against cancer cell growth was evaluated in various BCL cell lines in vitro. The cell was cultured for 4 days with increasing concentrations of CMC-544 in the presence of 20 ug/mL of rituximab.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 6.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Human B-lymphoma cells were cultured for 96 hours in the presence of various concentrations of CMC-544, CMA-676, or unconjugated CalichDMH after which the viable cell number in each culture was enumerated by their exclusion of propidium iodide and detected by flow cytometry.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | RL cells | CVCL_1660 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 6.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various ALL or B-NHL cell lines in vitro. The cell was examined in 96h in vitro culture assays.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | RL cells | CVCL_1660 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 13.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various ALL or B-NHL cell lines in vitro. The cell was examined in 96h in vitro culture assays.
|
||||
| In Vitro Model | B acute lymphoblastic leukemia | Reh cells | CVCL_1650 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.00 pM
|
|||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various ALL or B-NHL cell lines in vitro. The cell was examined in 96h in vitro culture assays.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
21.00 pM
|
|||
| Method Description |
Human B-lymphoma cells were cultured for 96 hours in the presence of various concentrations of CMC-544, CMA-676, or unconjugated CalichDMH after which the viable cell number in each culture was enumerated by their exclusion of propidium iodide and detected by flow cytometry.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 40.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 combining with rituximab against cancer cell growth was evaluated in various BCL cell lines in vitro. The cell was cultured for 4 days with increasing concentrations of CMC-544 in the presence of 100 ug/mL of rituximab.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 47.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various ALL or B-NHL cell lines in vitro. The cell was examined in 96h in vitro culture assays.
|
||||
| In Vitro Model | Adult B acute lymphoblastic leukemia | RS4;11 cells | CVCL_0093 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 53.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various ALL or B-NHL cell lines in vitro. The cell was examined in 96h in vitro culture assays.
|
||||
| In Vitro Model | B-lymphoblastic leukemia | SUP-B15 cells | CVCL_0103 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 53.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 combining with rituximab against cancer cell growth was evaluated in various BCL cell lines in vitro. The cell was cultured for 4 days with increasing concentrations of CMC-544 in the presence of 20 ug/mL of rituximab.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 80.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various ALL or B-NHL cell lines in vitro. The cell was examined in 96h in vitro culture assays.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 100.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various ALL or B-NHL cell lines in vitro. The cell was examined in 96h in vitro culture assays.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 200.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Human B-lymphoma cells were cultured for 96 hours in the presence of various concentrations of CMC-544, CMA-676, or unconjugated CalichDMH after which the viable cell number in each culture was enumerated by their exclusion of propidium iodide and detected by flow cytometry.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
250.00 pM
|
|||
| Method Description |
Human B-lymphoma cells were cultured for 96 hours in the presence of various concentrations of CMC-544, CMA-676, or unconjugated CalichDMH after which the viable cell number in each culture was enumerated by their exclusion of propidium iodide and detected by flow cytometry.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 300.00 pM | Negative CD22 expression (CD22-); Positive CD33 expression (CD33+++/++) | ||
| Method Description |
Human B-lymphoma cells were cultured for 96 hours in the presence of various concentrations of CMC-544, CMA-676, or unconjugated CalichDMH after which the viable cell number in each culture was enumerated by their exclusion of propidium iodide and detected by flow cytometry.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [13] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 600.00 pM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 combining with rituximab against cancer cell growth was evaluated in various BCL cell lines in vitro. The cell was cultured for 4 days with increasing concentrations of CMC-544 in the presence of 20 ug/mL of rituximab.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | RL cells | CVCL_1660 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
750.00 pM
|
|||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various ALL or B-NHL cell lines in vitro. The cell was examined in 96h in vitro culture assays.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.02 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various B-lymphoma cell lines in vitro. BCL cells were cultured in the presence of increasing concentrations of drugs and, after 96 h, the number of surviving live cells in culture was enumerated using the MTS assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.10 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various B-lymphoma cell lines in vitro. BCL cells were cultured in the presence of increasing concentrations of drugs and, after 96 h, the number of surviving live cells in culture was enumerated using the MTS assay.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | RL cells | CVCL_1660 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.20 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various B-lymphoma cell lines in vitro. BCL cells were cultured in the presence of increasing concentrations of drugs and, after 96 h, the number of surviving live cells in culture was enumerated using the MTS assay.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [14] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.50 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of CMC-544 against cancer cell growth was evaluated in various B-lymphoma cell lines in vitro. BCL cells were cultured in the presence of increasing concentrations of drugs and, after 96 h, the number of surviving live cells in culture was enumerated using the MTS assay.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | SU-DHL-4 cells | CVCL_0539 | ||
References
