Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0SMEOG
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Rituximab-Compound (la) DAR 8
|
|||||
| Synonyms |
Rituximab Compound (la)
Click to Show/Hide
|
|||||
| Organization |
Orum Therapeutics, Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
8
|
|||||
| Structure |
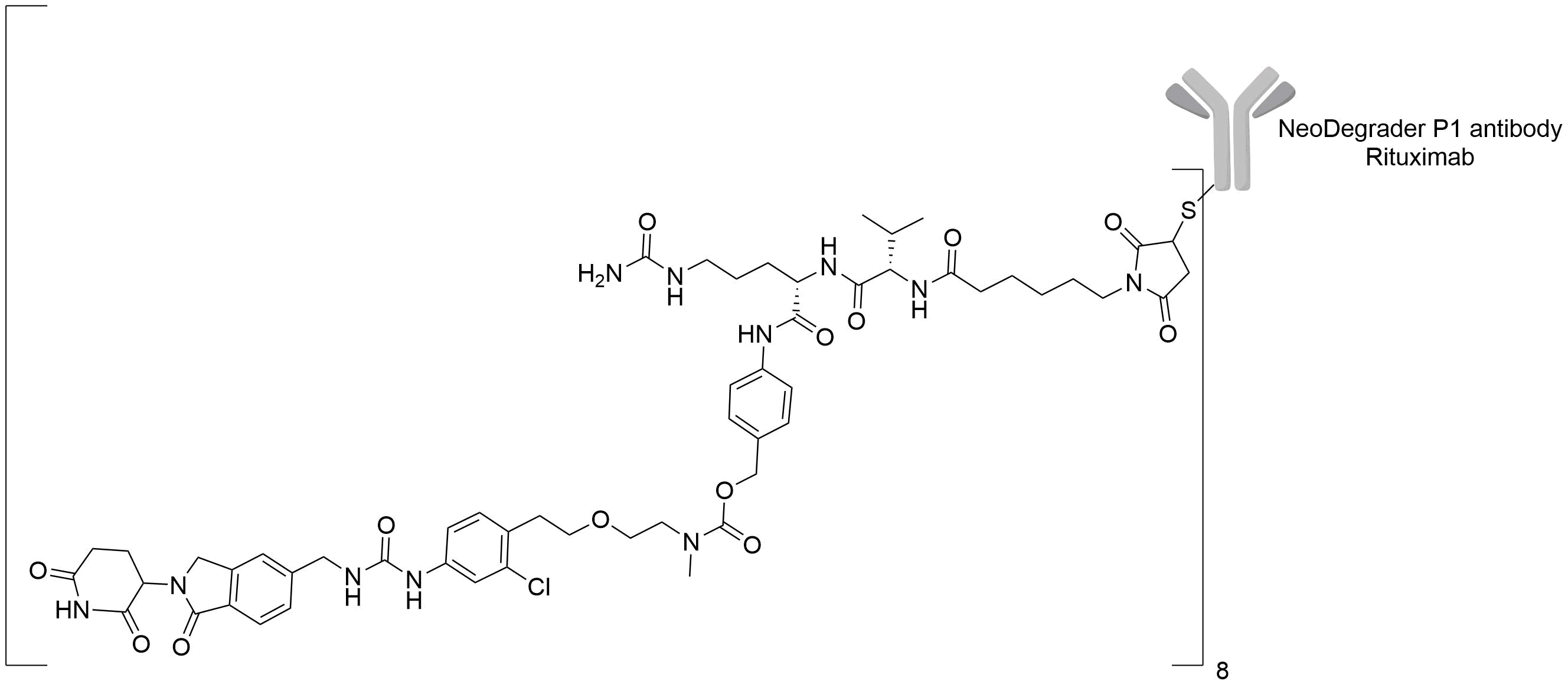
|
|||||
| Antibody Name |
Rituximab
|
Antibody Info | ||||
| Antigen Name |
B-lymphocyte antigen CD20 (MS4A1)
|
Antigen Info | ||||
| Payload Name |
NeoDegrader P1
|
Payload Info | ||||
| Therapeutic Target |
Protein cereblon (CRBN)
|
Target Info | ||||
| Linker Name |
Rituximab-Compound (la) linker
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 42) | Positive CD20 expression (CD20+++/++) | ||
| Method Description |
Daudi human NHL fragments were implanted subcutaneously in theright flank of the mice. Mice were divided into 4 treatment groups (N=8/group), as follows: trastuzumab-Compound (la) conjugate (5 mg/kg, iv, qd x 1); rituximab-Compound (la) conjugate (1 mg/kg, iv, qd x 1).
|
||||
| In Vivo Model | Daudi CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 56.79% (Day 42) | Positive CD20 expression (CD20+++/++) | ||
| Method Description |
Daudi human NHL fragments were implanted subcutaneously in theright flank of the mice. Mice were divided into 4 treatment groups (N=8/group), as follows: trastuzumab-Compound (la) conjugate (5 mg/kg, iv, qd x 1); rituximab-Compound (la) conjugate (5 mg/kg, iv, qd x 1).
|
||||
| In Vivo Model | Daudi CDX model | ||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 84.72% (Day 30) | High HER2 expression (HER2+++) | ||
| Method Description |
BT474 human breast carcinomas fragments were implanted subcutaneously in theright flank of the mice. Mice were divided into 4 treatment groups (N=8/group), as follows: trastuzumab-Compound (la) conjugate (5 mg/kg, iv, qd x 1); rituximab-Compound (la) conjugate (5 mg/kg, iv, qd x 1); pertuzumab-Compound (la) conjugate (5 mg/kg, iv, qd x 1).
|
||||
| In Vivo Model | BT-474 CDX model | ||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.10 nM | Positive CD20 expression (CD20+++/++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.44 nM | Positive CD20 expression (CD20+++/++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
|||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Adult hepatocellular carcinoma | SNU-182 cells | CVCL_0090 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | |||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Endometrial adenocarcinoma | JHUEM-3 cells | CVCL_4657 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | |||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H2030 cells | CVCL_1517 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | |||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | CFPAC-1 cells | CVCL_1119 | ||
