Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0PXYTA
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
MEDI-4276
|
|||||
| Synonyms |
MEDI 4276; MEDI-4276; MEDI4 276; MEDI4276
Click to Show/Hide
|
|||||
| Organization |
MedImmune LLC; AstraZeneca PLC
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
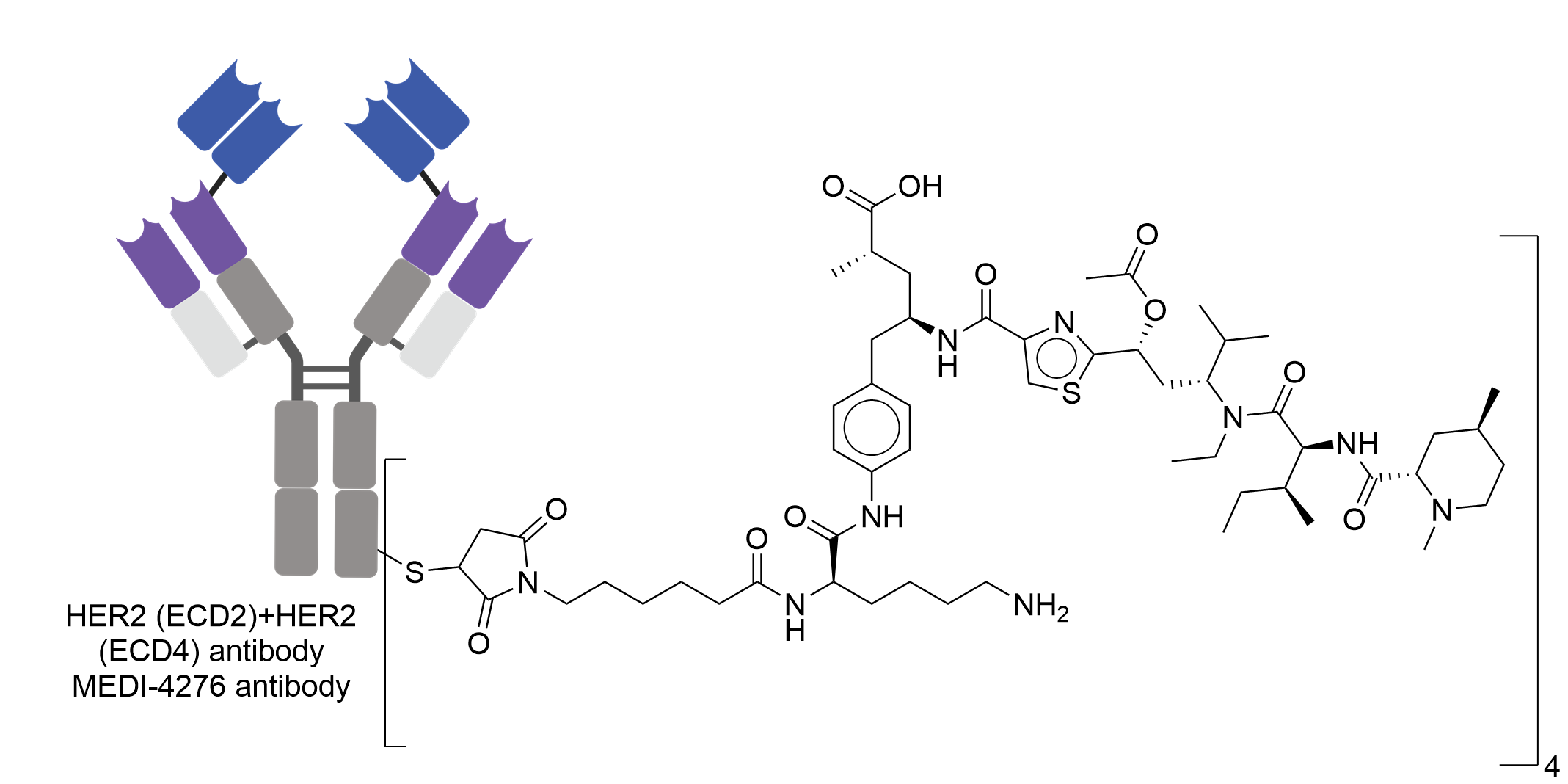
|
|||||
| Antibody Name |
MEDI-4276
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2 ECD2); Receptor tyrosine-protein kinase erbB-2 (HER2 ECD4)
|
Antigen Info | ||||
| Payload Name |
AZ13599185
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Maleimido-caproyl based linker
|
Linker Info | ||||
| Conjugate Type |
Reactive Cysteines
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
