Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0PPHVA
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Trastuzumab-SYNtansine
|
|||||
| Synonyms |
Trastuzumab-BCN-HydraSpace-vc-PABC-Ahx-maytansine
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.7
|
|||||
| Structure |
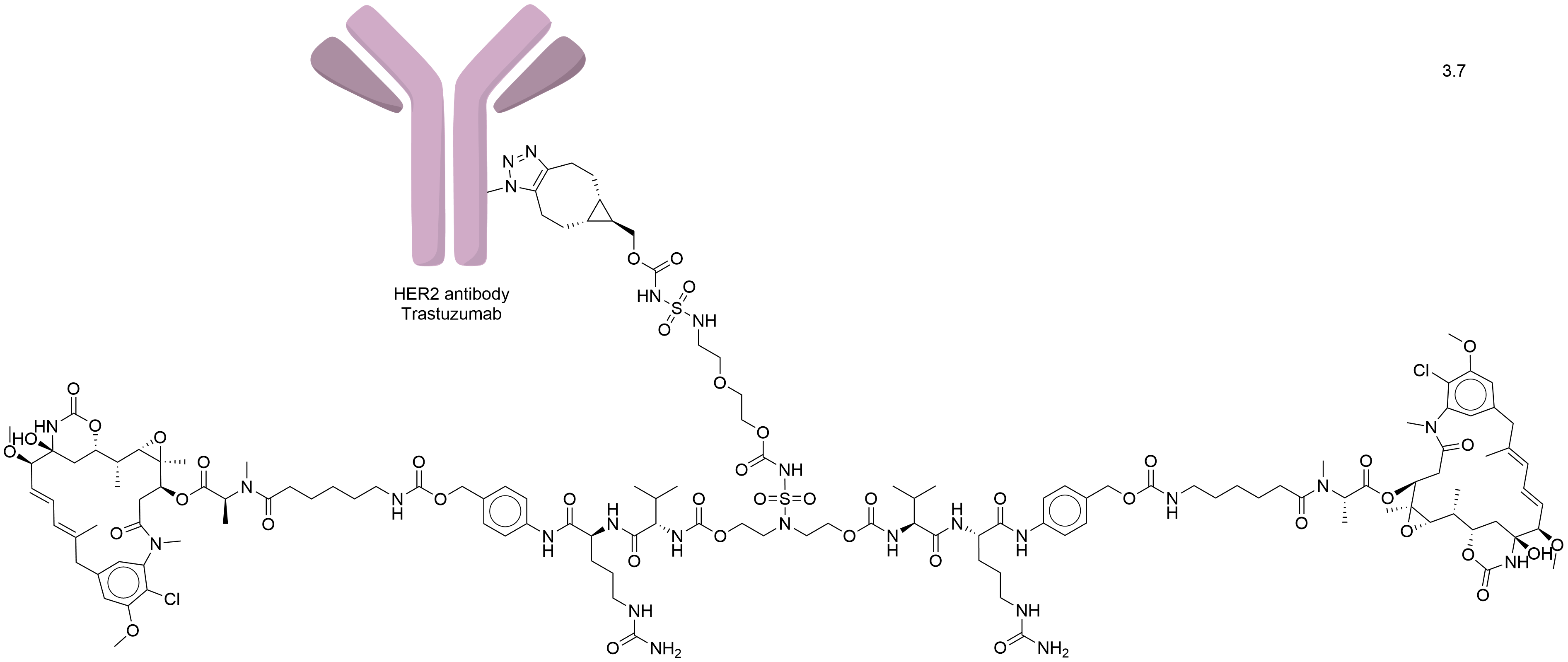
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Ahx-maytansine
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
BCN-HydraSpace-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
GlycoConnect technology uses the globally conserved N-glycosylation site (Asn 279) to generate stable and site-specific ADCs based on enzymatic remodeling (engineered endoglycosidase and native glycosyl transferase).
|
|||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.60% (Day 23) | High HER2 expression (HER2+++) | ||
| Method Description |
25 mice with T226 tumor (P12.1.4/0) between 75 and 196 mm3 were allocated, according to their tumor volume to give homogenous mean and median tumor volume in each treatment arm (5 mice/group). Treatments were initiated when the median tumor volume was 126 mm3 by intravenous injection with either vehicle (control), and trastuzumab-SYNtansine 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: T226) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 23) | High HER2 expression (HER2+++) | ||
| Method Description |
25 mice with T226 tumor (P12.1.4/0) between 75 and 196 mm3 were allocated, according to their tumor volume to give homogenous mean and median tumor volume in each treatment arm (5 mice/group). Treatments were initiated when the median tumor volume was 126 mm3 by intravenous injection with either vehicle (control), and trastuzumab-SYNtansine 9 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: T226) | ||||
References
