Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0NYFRQ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Atezolizumab ADC
|
|||||
| Synonyms |
Bifunctional PD-L1 ADC (ADC 3); Atezolizumab ADC 3
Click to Show/Hide
|
|||||
| Organization |
Beijing Institute of Pharmacology and Toxicology
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4.8
|
|||||
| Structure |
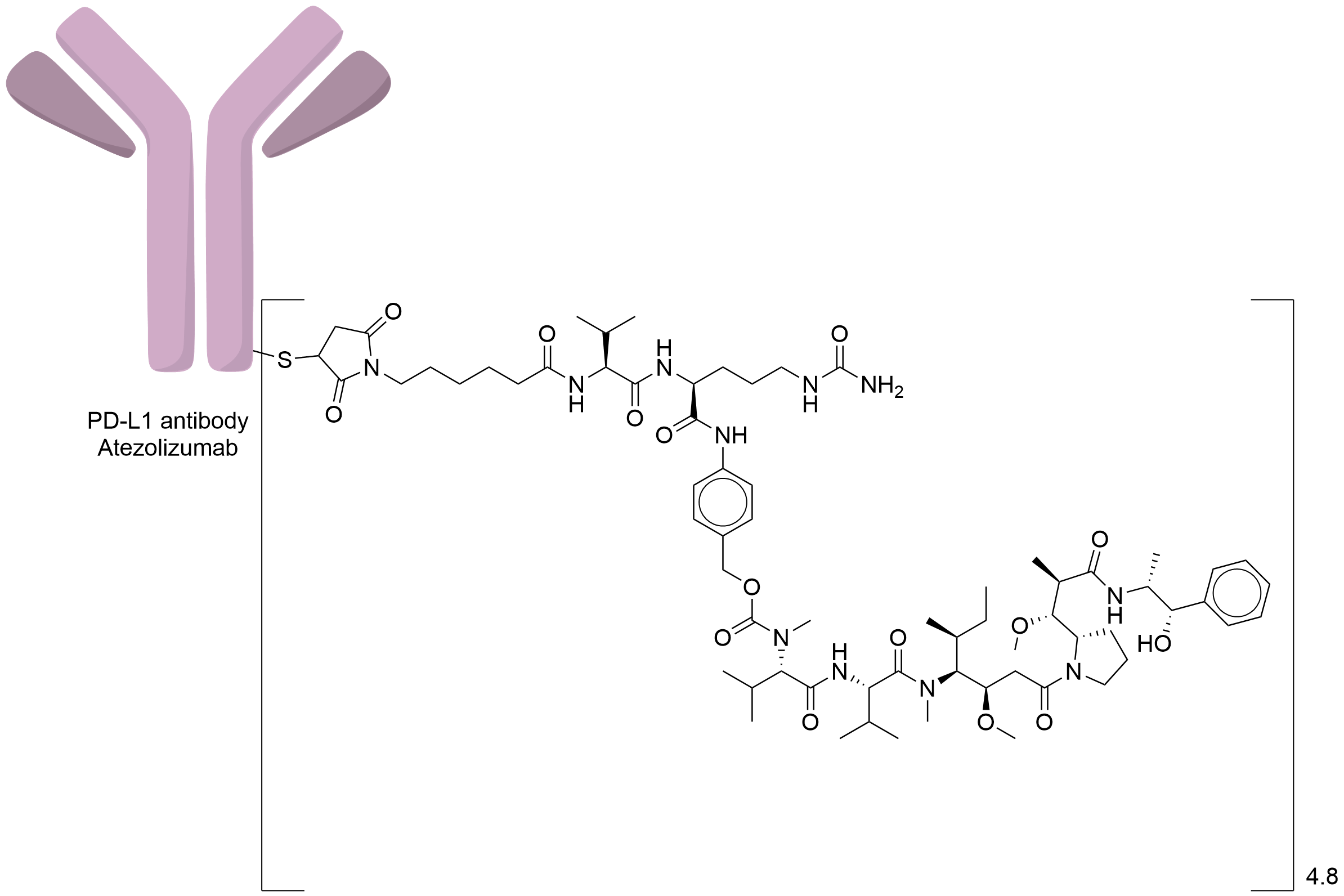
|
|||||
| Antibody Name |
Atezolizumab
|
Antibody Info | ||||
| Antigen Name |
Programmed cell death 1 ligand 1 (CD274)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
