Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0LXXFO
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
BVD-TEM8 ADC
|
|||||
| Synonyms |
BVD TEM8 ADC; BVDTEM8 ADC; m825-MMAE
Click to Show/Hide
|
|||||
| Organization |
BioMed Valley Discoveries, Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 5 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
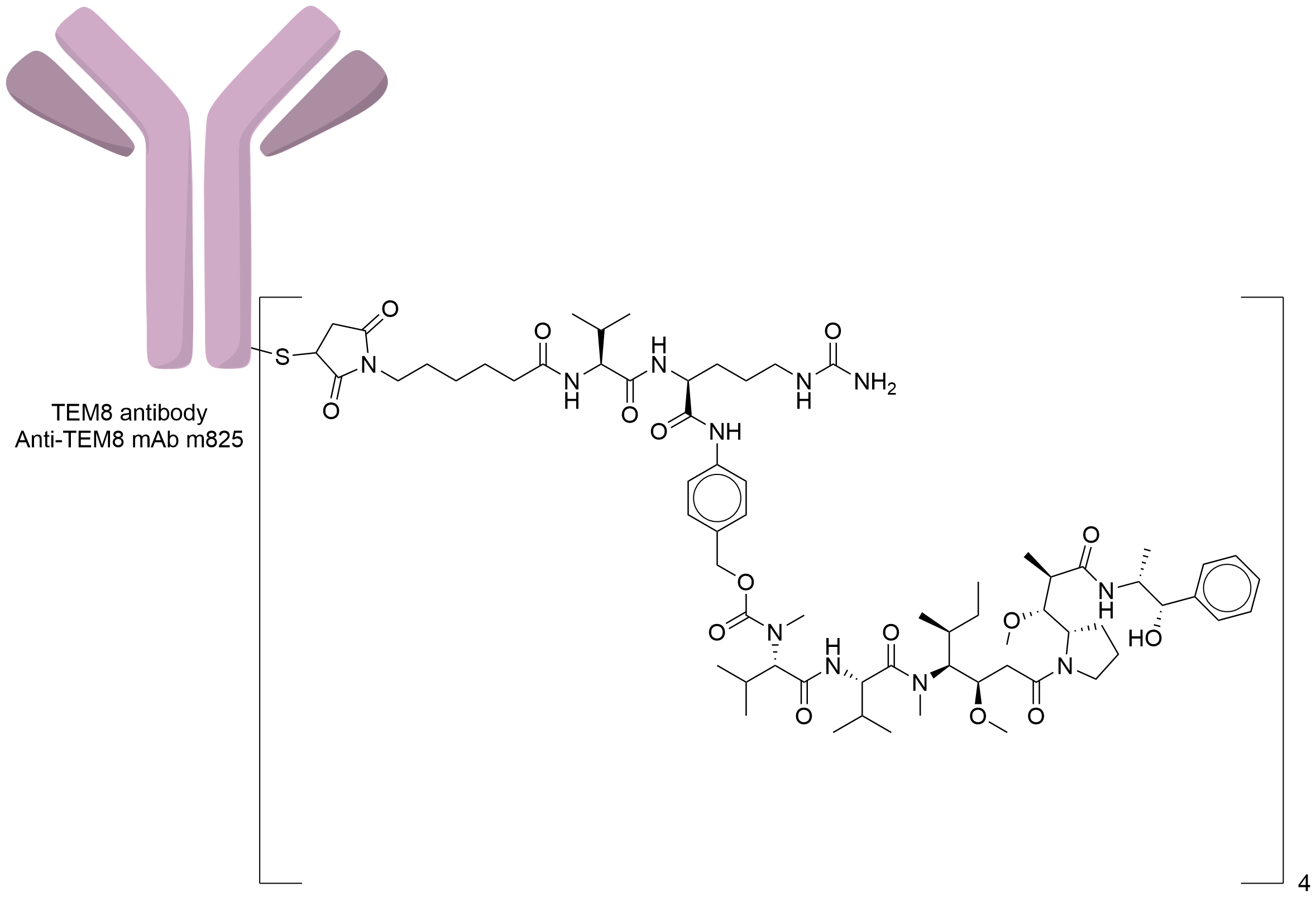
|
|||||
| Antibody Name |
Anti-TEM8 mAb m825
|
Antibody Info | ||||
| Antigen Name |
Anthrax toxin receptor 1 (ANTXR1)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through reduced inter-chain cysteines.
|
|||||
| Combination Type |
Vedotin
|
|||||
