Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0KYHQN
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
CMB-401
|
|||||
| Synonyms |
Anti-PEM monoclonal antibody calicheamicin; CDP 671; CDP-671; CMB 401; CMB-401; LLE 33288; hCTMO1-calicheamicin; hcT.MO1 calicheamicin
Click to Show/Hide
|
|||||
| Organization |
Celltech Oy
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2 to 3
|
|||||
| Structure |
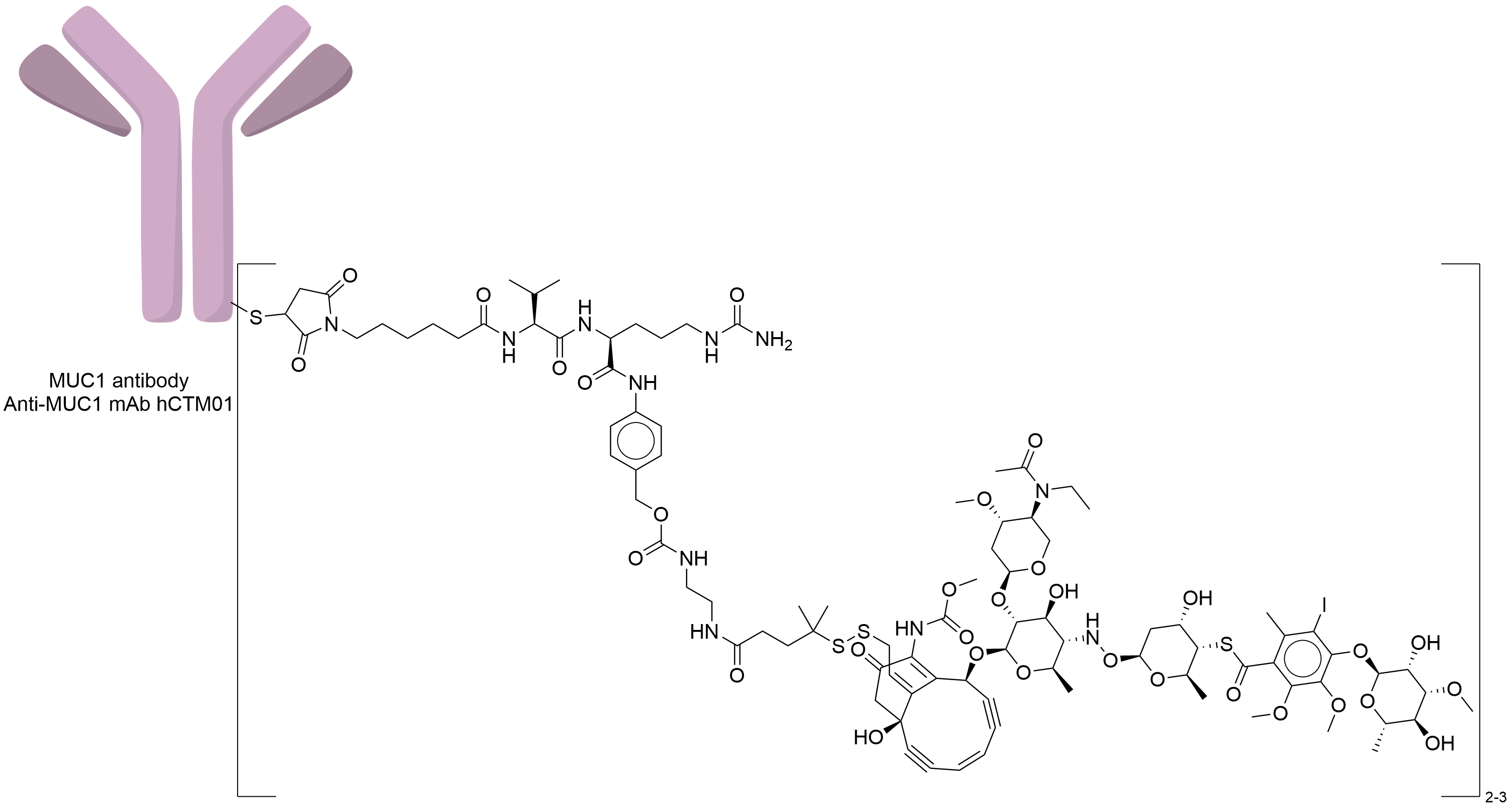
|
|||||
| Antibody Name |
Anti-MUC1 mAb hCTM01
|
Antibody Info | ||||
| Antigen Name |
Mucin-1 (MUC1)
|
Antigen Info | ||||
| Payload Name |
N-acetyl-gamma-calicheamicin
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Amide-based linker
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Puchem SID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Platinum-sensitive EOC (recurrence >6 months after completion of an initial platinumcontaining chemotherapy regimen).
|
||||
| Administration Dosage |
Initial infusion of 35 mg/m2 of hCTM01 (anti-PEM antibody not conjugated to calicheamicin) over a period of 15 min, followed 45 min later (60 min from the start of the initial infusion) by an infusion of 16 mg/m2 of CMB-401 over an additional 60 min, up to 7 cycles, with 4 weeks between cycles.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Patients Enrolled |
EOC refractory to or unsuitable for platinum/standard therapy; with a WHO performance status of 0-2; life expectancy of more than three months and fulfilling other standard eligibility criteria.
|
||||
| Administration Dosage |
Up to four cycles of a dual infusion of 35.00 mg/m2 hCTMO1 'predose' followed by doses of CMB-401 which were increased for each cohort, CMB-401 dosing commenced at 2 mg/m2 and progressed via seven cohorts to 16.00 mg/m2.
|
||||
References
