Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0KWKQC
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
DX126-262
|
|||||
| Synonyms |
DAC-001; DX126 262; DX126-262; Recombinant anti-HER2 antibody-Tub 114
Click to Show/Hide
|
|||||
| Organization |
Hangzhou DAC Biotech Co., Ltd.
|
|||||
| Drug Status |
Phase 2
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.5 to 3.8
|
|||||
| Structure |
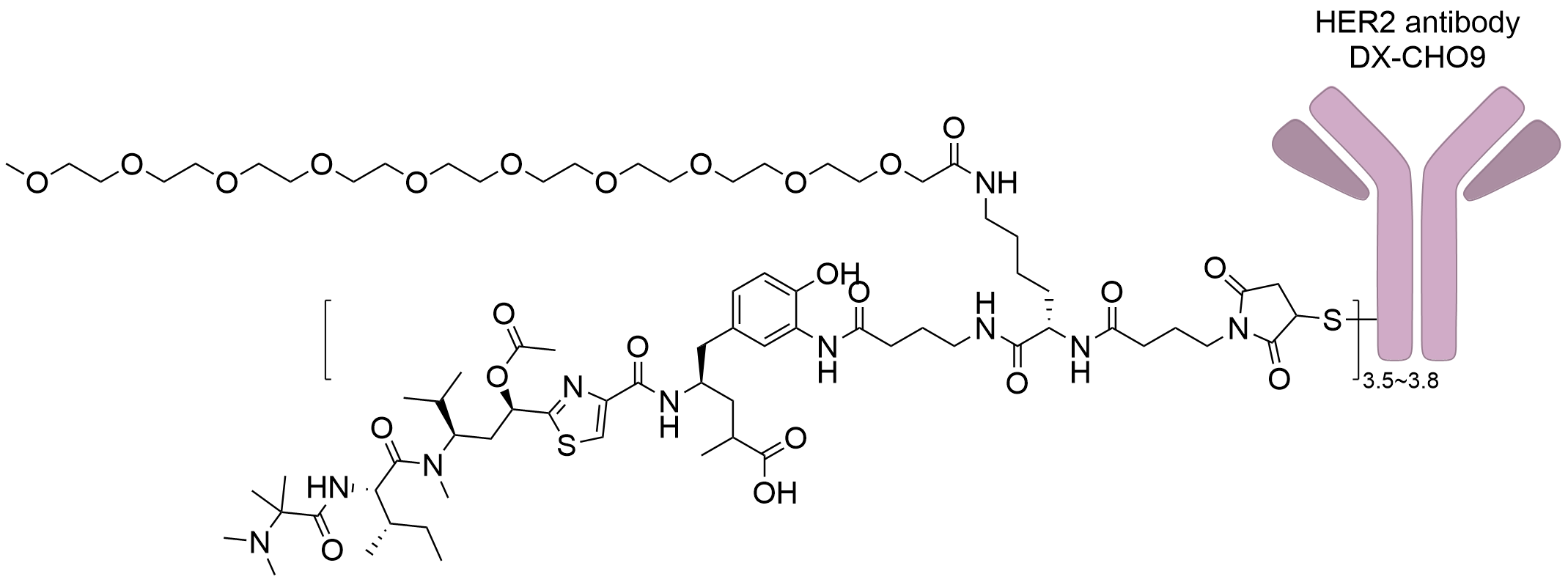
|
|||||
| Antibody Name |
DX-CHO9
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Tubulysin B analog Tub114
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mal-PEG10-Ala-Ala-linker
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Special Approval(s) |
Special approval (NMPA)
|
|||||
