Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0JYNCF
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
CX-2029
|
|||||
| Synonyms |
ABBV-2029; ABBV-2029,CX-2029; CD71 probody drug conjugate; CX 2029; CX-2029
Click to Show/Hide
|
|||||
| Organization |
CytomX Therapeutics, Inc.; AbbVie, Inc.
|
|||||
| Drug Status |
Phase 1/2
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
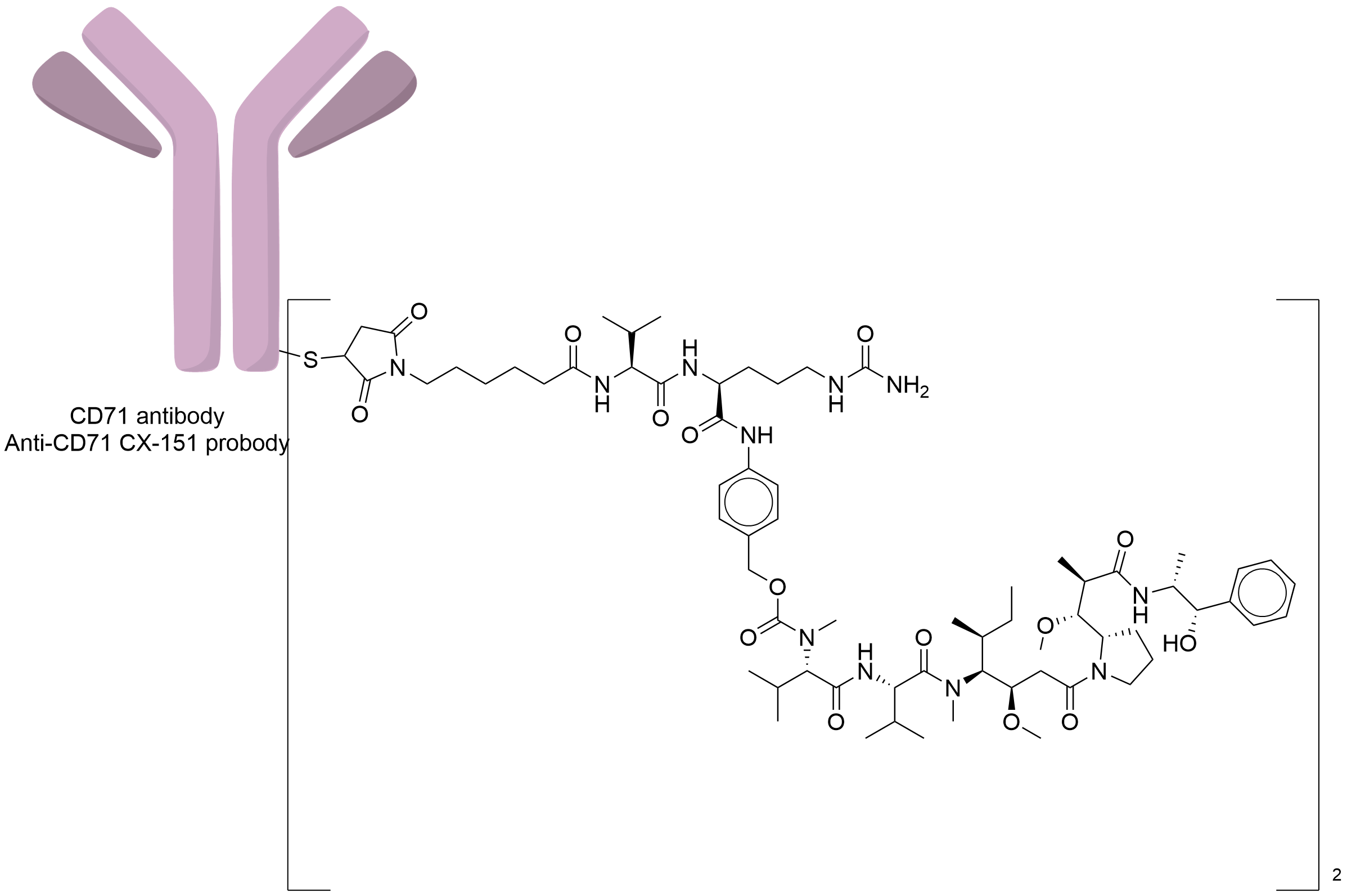
|
|||||
| Antibody Name |
Anti-CD71 CX-151 probody
|
Antibody Info | ||||
| Antigen Name |
Transferrin receptor protein 1 (TFRC)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
vedotin
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) | 20.00% (dose of 3 mg/kg), 50.00% (dose of 5 mg/kg) | High CD71 expression (CD71+++) | ||
| Patients Enrolled |
Metastatic or locally advanced unresectable solid tumors without approved life-prolonging treatment options.
|
||||
| Administration Dosage |
CX-2029 (0.10, 0.25, 0.50, 1, 2, 3, 4, or 5 mg/kg) i.v. over 90 minutes [later increased to 180 minutes to mitigate infusion-related reactions (IRR)] every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03543813 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1-2, first-in-human study of CX-2029 in adults with metastatic or locally advanced unresectable solid tumors or diffuse large B-cell lymphomas (PROCLAIM-CX-2029). | ||||
| Primary Endpoint |
For the dose of 3 mg/kg, confirmed partial response rate=20.00% (n=2, 95% CI=2.50-55.60). For the dose of 5 mg/kg, confirmed partial response rate=50.00% (n=1, 95% CI=1.30-98.70).
|
||||
| Other Endpoint |
For the dose of 0.5 mg/kg, Disease controla rate=50.00% (n=3, 95% CI=11.80-82.20). For the dose of 2 mg/kg, Disease controla rate=28.60% (n=2, 95% CI=3.7-71.0). For the dose of 3 mg/kg, Disease controla rate=50.00% (n=5, 95% CI 18.70-81.30). For the dose of 5 mg/kg, Disease controla rate=50.00% (n=1, 95% CI 1.30-98.70).
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 74.10% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 3 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PA6237) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.80% (Day 18) | High CD71 expression (CD71+++) | ||
| Method Description |
Female BALB/c nude mice used for esophageal,gastric,pancreatic models,and NOD/SCID,NPG,or NOG mice used for DLBCL models and used at ages 5 to 7 weeks. Seven- to 8-week-old female Athymic Nude-Foxn1nu mice were used for breast,HNSCC,and NSCLC models (Envigo). Human cancer cell lines or tumor fragments 2 to 3 mm in diameter from stock mice inoculated with primary human tumor xenografts were harvested and used to inoculate study mice by subcutaneous injection into the right flank. When tumors reached approximately 100 to 200 mm3,mice were randomized into treatment groups based on tumor volume and body weight. Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS),1.5 mg/kg,3 mg/kg,or 6 mg anti-CD71 PDC on day 0 and day 9. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
Click to Show/Hide
|
||||
| In Vivo Model | CTG-820 PDX model | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.60% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 6 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Non-small cell lung cancer PDX model (PDX: CTG-0860) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.00% (Day 32) | High CD71 expression (CD71+++) | ||
| Method Description |
Female BALB/c nude mice used for esophageal,gastric,pancreatic models,and NOD/SCID,NPG,or NOG mice used for DLBCL models and used at ages 5 to 7 weeks. Seven- to 8-week-old female Athymic Nude-Foxn1nu mice were used for breast,HNSCC,and NSCLC models (Envigo). Human cancer cell lines or tumor fragments 2 to 3 mm in diameter from stock mice inoculated with primary human tumor xenografts were harvested and used to inoculate study mice by subcutaneous injection into the right flank. When tumors reached approximately 100 to 200 mm3,mice were randomized into treatment groups based on tumor volume and body weight. Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS),1.5 mg/kg,3 mg/kg,or 6 mg anti-CD71 PDC on day 0 and day 10. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
Click to Show/Hide
|
||||
| In Vivo Model | CTG-820 PDX model | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.00% (Day 18) | High CD71 expression (CD71+++) | ||
| Method Description |
Female BALB/c nude mice used for esophageal,gastric,pancreatic models,and NOD/SCID,NPG,or NOG mice used for DLBCL models and used at ages 5 to 7 weeks. Seven- to 8-week-old female Athymic Nude-Foxn1nu mice were used for breast,HNSCC,and NSCLC models (Envigo). Human cancer cell lines or tumor fragments 2 to 3 mm in diameter from stock mice inoculated with primary human tumor xenografts were harvested and used to inoculate study mice by subcutaneous injection into the right flank. When tumors reached approximately 100 to 200 mm3,mice were randomized into treatment groups based on tumor volume and body weight. Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS),1.5 mg/kg,3 mg/kg,or 6 mg anti-CD71 PDC on day 0 and day 13. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
Click to Show/Hide
|
||||
| In Vivo Model | CTG-820 PDX model | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.40% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 3 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA6881) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.80% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 3 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: CTG-0708) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 6 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PA6237) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 6 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Diffuse large B-cell lymphoma PDX model (PDX: LY6934) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 6 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Head and neck squamous cell carcinoma PDX model (PDX: CTG-820) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 6 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: CTG-0708) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 6 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: GA6881) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | High CD71 expression (CD71+++) | ||
| Method Description |
Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS) , 6 mg/kg on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
|
||||
| In Vivo Model | Esophageal caner PDX model (PDX: ES0136) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 21) | High CD71 expression (CD71+++) | ||
| Method Description |
Female BALB/c nude mice used for esophageal,gastric,pancreatic models,and NOD/SCID,NPG,or NOG mice used for DLBCL models and used at ages 5 to 7 weeks. Seven- to 8-week-old female Athymic Nude-Foxn1nu mice were used for breast,HNSCC,and NSCLC models (Envigo). Human cancer cell lines or tumor fragments 2 to 3 mm in diameter from stock mice inoculated with primary human tumor xenografts were harvested and used to inoculate study mice by subcutaneous injection into the right flank. When tumors reached approximately 100 to 200 mm3,mice were randomized into treatment groups based on tumor volume and body weight. Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS),1.5 mg/kg,3 mg/kg,or 6 mg anti-CD71 PDC on day 0 and day 7. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PA6237) | ||||
| Experiment 15 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 18) | High CD71 expression (CD71+++) | ||
| Method Description |
Female BALB/c nude mice used for esophageal,gastric,pancreatic models,and NOD/SCID,NPG,or NOG mice used for DLBCL models and used at ages 5 to 7 weeks. Seven- to 8-week-old female Athymic Nude-Foxn1nu mice were used for breast,HNSCC,and NSCLC models (Envigo). Human cancer cell lines or tumor fragments 2 to 3 mm in diameter from stock mice inoculated with primary human tumor xenografts were harvested and used to inoculate study mice by subcutaneous injection into the right flank. When tumors reached approximately 100 to 200 mm3,mice were randomized into treatment groups based on tumor volume and body weight. Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS),1.5 mg/kg,3 mg/kg,or 6 mg anti-CD71 PDC on day 0 and day 8. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
Click to Show/Hide
|
||||
| In Vivo Model | Diffuse large B-cell lymphoma PDX model (PDX: LY6934) | ||||
| Experiment 16 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 36) | High CD71 expression (CD71+++) | ||
| Method Description |
Female BALB/c nude mice used for esophageal,gastric,pancreatic models,and NOD/SCID,NPG,or NOG mice used for DLBCL models and used at ages 5 to 7 weeks. Seven- to 8-week-old female Athymic Nude-Foxn1nu mice were used for breast,HNSCC,and NSCLC models (Envigo). Human cancer cell lines or tumor fragments 2 to 3 mm in diameter from stock mice inoculated with primary human tumor xenografts were harvested and used to inoculate study mice by subcutaneous injection into the right flank. When tumors reached approximately 100 to 200 mm3,mice were randomized into treatment groups based on tumor volume and body weight. Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS),1.5 mg/kg,3 mg/kg,or 6 mg anti-CD71 PDC on day 0 and day 11. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: GA6881) | ||||
| Experiment 17 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 40) | High CD71 expression (CD71+++) | ||
| Method Description |
Female BALB/c nude mice used for esophageal,gastric,pancreatic models,and NOD/SCID,NPG,or NOG mice used for DLBCL models and used at ages 5 to 7 weeks. Seven- to 8-week-old female Athymic Nude-Foxn1nu mice were used for breast,HNSCC,and NSCLC models (Envigo). Human cancer cell lines or tumor fragments 2 to 3 mm in diameter from stock mice inoculated with primary human tumor xenografts were harvested and used to inoculate study mice by subcutaneous injection into the right flank. When tumors reached approximately 100 to 200 mm3,mice were randomized into treatment groups based on tumor volume and body weight. Dosing started on the same day for all groups,and dosing volume was adjusted for each mouse based on body weight on day of dosing. PDX models were intravenously dosed with vehicle (PBS),1.5 mg/kg,3 mg/kg,or 6 mg anti-CD71 PDC on day 0 and day 12. Mice were checked daily for morbidity and mortality. Tumors were measured twice weekly via calipers.
Click to Show/Hide
|
||||
| In Vivo Model | Esophageal cancer PDX model (PDX: ES0136) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.20 nM
|
|||
| Method Description |
Suspension or adherent cells were plated at a density of 1, 000 cells/well in 50-mL complete media in a 96-well white-walled tissue culture plate and used immediately or allowed to adhere overnight. Cells were then incubated with 50 uL of a 2 final concentration of test article for 3 to 5 days at 37 and 5% CO2.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H520 cells | CVCL_1566 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 1.30 nM | High CD71 expression (CD71+++) | ||
| Method Description |
Suspension or adherent cells were plated at a density of 1, 000 cells/well in 50-mL complete media in a 96-well white-walled tissue culture plate and used immediately or allowed to adhere overnight. Cells were then incubated with 50 uL of a 2 final concentration of test article for 3 to 5 days at 37 and 5% CO2.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
References
