Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0JMIOG
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
LSR-ADC
|
|||||
| Synonyms |
LSR ADC
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2.8
|
|||||
| Structure |
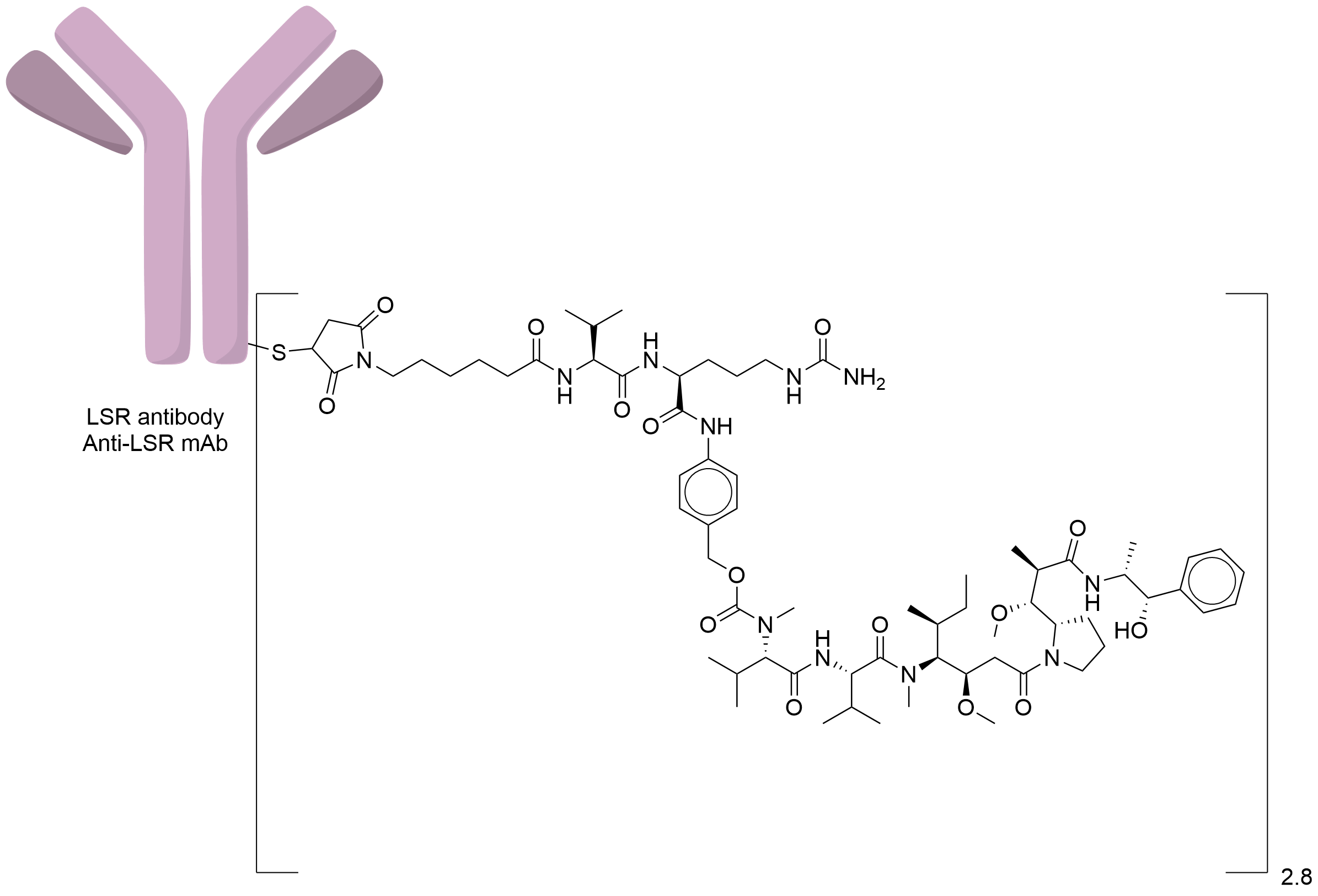
|
|||||
| Antibody Name |
Anti-LSR mAb
|
Antibody Info | ||||
| Antigen Name |
Lipolysis-stimulated lipoprotein receptor (LSR)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through reduced inter-chain cysteines.
|
|||||
| Combination Type |
Vedotin
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Patient-derived Xenograft Model
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.53% (Day 70) | High LSR expression (LSR+++; 89,382.9 LSR expression (ABC/cell)) | ||
| Method Description |
The model was Ovx6 PDX,which was generated by implanting human tumor tissues expressing LSR at high levels. When the mean tumor size of each cancer type reached approximately 110 mm3,PBS or LSR-ADC (1 mg/kg) were intravenously injected twice a week,for a total of four times.
|
||||
| In Vivo Model | Ovarian cancer PDX model (PDX: Ovx6) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.47% (Day 70) | High LSR expression (LSR+++; 89,382.9 LSR expression (ABC/cell)) | ||
| Method Description |
The model was Ovx6 PDX,which was generated by implanting human tumor tissues expressing LSR at high levels. When the mean tumor size of each cancer type reached approximately 110 mm3,PBS or LSR-ADC (3 mg/kg) were intravenously injected twice a week,for a total of four times.
|
||||
| In Vivo Model | Ovarian cancer PDX model (PDX: Ovx6) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 67.50% (Day 21) | High LSR expression (LSR+++; 89,382.9 LSR expression (ABC/cell)) | ||
| Method Description |
To assess the efficacy of LSR-ADC against omental/bowel metastasis,OVCAR3-Luc xenograft models were used. The OVCAR3-Luc xenograft models was established by implanting OVCAR3-Luc cells intraperitoneally,into the subscapular areas. Six or seven weeks after the inoculation,the treatment was initiated. PBS and 10 mg/kg of LSR-ADC were intravenously injected twice a week,for a total of four times.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian high-grade serous carcinoma PDX model (PDX: OVCAR3-LUC) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.24% (Day 70) | High LSR expression (LSR+++; 86,697.7 LSR expression (ABC/cell)) | ||
| Method Description |
The model was Ovx6 PDX,which was generated by implanting human tumor tissues expressing LSR at high levels. When the mean tumor size of each cancer type reached approximately 110 mm3,PBS or LSR-ADC (10 mg/kg) were intravenously injected twice a week,for a total of four times.
|
||||
| In Vivo Model | Ovarian cancer PDX model (PDX: Ovx6) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 21.41% (Day 28) | High LSR expression (LSR+++) | ||
| Method Description |
The model was established by subcutaneously implanting OVCAR3 cells into CB17/SCID mice. When the mean tumor size of each cancer type reached approximately 110 mm3,PBS or LSR-ADC (1 mg/kg) were intravenously injected twice a week,for a total of four times.
|
||||
| In Vivo Model | Ovarian high-grade serous carcinoma CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.43% (Day 28) | High LSR expression (LSR+++) | ||
| Method Description |
The model was established by subcutaneously implanting OVCAR3 cells into CB17/SCID mice. When the mean tumor size of each cancer type reached approximately 110 mm3,PBS or LSR-ADC (3 mg/kg) were intravenously injected twice a week,for a total of four times.
|
||||
| In Vivo Model | Ovarian high-grade serous carcinoma CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.01% (Day 28) | High LSR expression (LSR+++) | ||
| Method Description |
The model was established by subcutaneously implanting OVCAR3 cells into CB17/SCID mice. When the mean tumor size of each cancer type reached approximately 110 mm3,PBS or LSR-ADC (10 mg/kg) were intravenously injected twice a week,for a total of four times.
|
||||
| In Vivo Model | Ovarian high-grade serous carcinoma CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 449.00 pM | High LSR expression (LSR+++; 89,382.9 LSR expression (ABC/cell)) | ||
| Method Description |
Cytotoxicity assays were performed in the presence of the anti-LSR mAb (#16-6),the mouse IgG2a isotype control antibody,the LSR-ADC or the control-ADC. After 24 h,the cells were incubated with serial dilutions of the agents in triplicate wells for 144 h,at 37°C,in a humidified 5% CO2 atmosphere. Cell viability was determined with the CellTiter-Glo Luminescent Cell Viability Assay Kit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.19 nM | High LSR expression (LSR+++; 86,697.7 LSR expression (ABC/cell)) | ||
| Method Description |
Cytotoxicity assays were performed in the presence of the anti-LSR mAb (#16-6),the mouse IgG2a isotype control antibody,the LSR-ADC or the control-ADC. After 24 h,the cells were incubated with serial dilutions of the agents in triplicate wells for 144 h,at 37°C,in a humidified 5% CO2 atmosphere. Cell viability was determined with the CellTiter-Glo Luminescent Cell Viability Assay Kit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 Luc cells | CVCL_0465 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 1.74 nM | High LSR expression (LSR+++; 84,008.8 LSR expression (ABC/cell)) | ||
| Method Description |
Cytotoxicity assays were performed in the presence of the anti-LSR mAb (#16-6),the mouse IgG2a isotype control antibody,the LSR-ADC or the control-ADC. After 24 h,the cells were incubated with serial dilutions of the agents in triplicate wells for 144 h,at 37°C,in a humidified 5% CO2 atmosphere. Cell viability was determined with the CellTiter-Glo Luminescent Cell Viability Assay Kit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian cancer ascites | NOVC-7C cells | Homo sapiens | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 uM | Moderate LSR expression (LSR++; 2,631.9 LSR expression (ABC/cell)) | ||
| Method Description |
Cytotoxicity assays were performed in the presence of the anti-LSR mAb (#16-6),the mouse IgG2a isotype control antibody,the LSR-ADC or the control-ADC. After 24 h,the cells were incubated with serial dilutions of the agents in triplicate wells for 144 h,at 37°C,in a humidified 5% CO2 atmosphere. Cell viability was determined with the CellTiter-Glo Luminescent Cell Viability Assay Kit.
Click to Show/Hide
|
||||
| In Vitro Model | Ewing sarcoma | ES2 cells | CVCL_AX39 | ||
References
