Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0IPCHO
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
TAK-164
|
|||||
| Synonyms |
TAK 164; TAK-164; TAK164
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.; Takeda Pharmaceutical Co., Ltd.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
Undisclosed
|
|||||
| Structure |
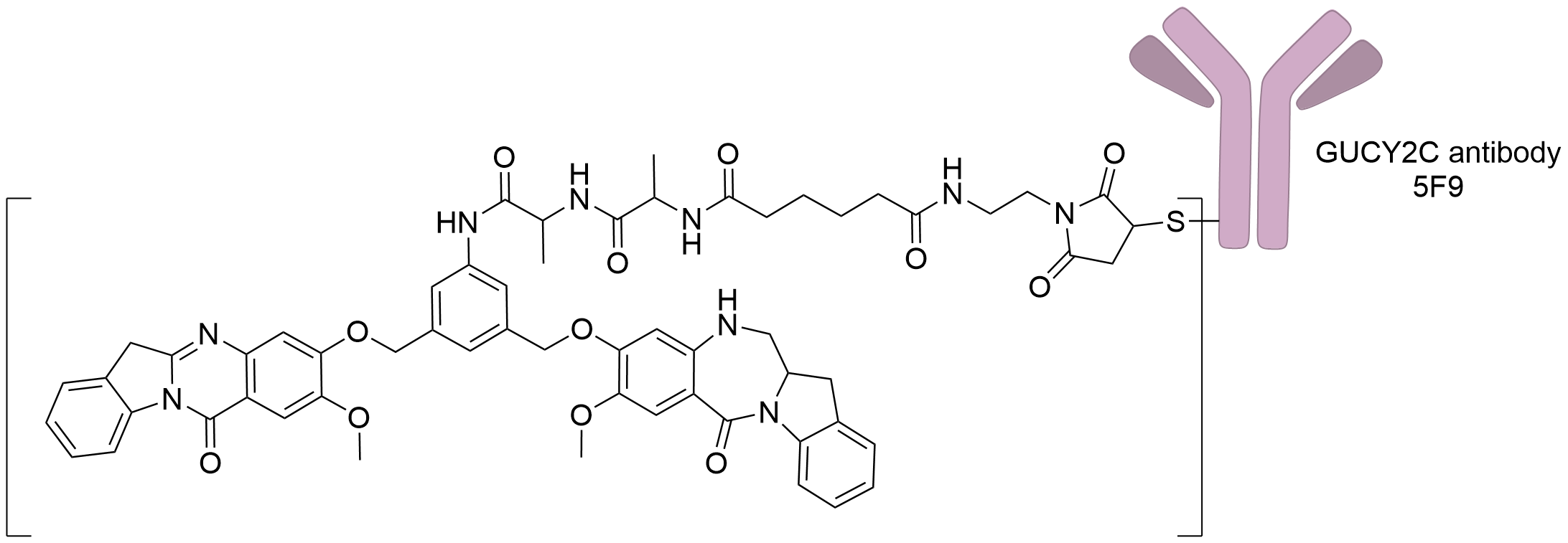
|
|||||
| Antibody Name |
5F9
|
Antibody Info | ||||
| Antigen Name |
Guanylyl cyclase C (GUCY2C)
|
Antigen Info | ||||
| Payload Name |
DGN549
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-adipamide-Ala-Ala
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
GCC-positive (H-score10 as indicated by IHC) GI cancers for whom standard treatment was no longer effective, or not available, Eligible GI malignancies included, but were not limited to: metastatic colorectal carcinoma, gastric carcinoma, esophageal carcinoma, small intestine cancer, and pancreatic cancer.
|
||||
| Administration Dosage |
TAK-164 as a single intravenous infusion with a duration of up to 2 h, on day 1 of each 21-day cycle or every 3 weeks, until disease progression, unacceptable toxicity, or withdrawal from the study. Doses of 0.004 mg/kg, 0.008 mg/kg, 0.016 mg/kg, 0.032 mg/kg, 0.064 mg/kg, 0.12 mg/kg, 0.16 mg/kg, 0.19 mg/kg, 0.25 mg/kg, and 0.32 mg/kg were planned. To guide dose escalation, a method based on a Bayesian model of modified toxicity probability interval (mTPI) was used.
Click to Show/Hide
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03449030 | Clinical Status | Phase 1 | ||
| Clinical Description | An open-label, dose escalation, phase 1, first-in-human study of TAK-164, an antibody-drug conjugate, in patients with advanced gastrointestinal cancers expressing guanylyl cyclase C. | ||||
| Primary Endpoint |
Dosing was capped at 0.19 mg/kg due to hepatic toxicity. The RP2D was determined as 0.064 mg/kg but was considered insufficient to derive significant clinical benefit.
|
||||
| Other Endpoint |
No pts had dose-limiting toxicities (DLT) in cycle 1 up to 0.32 mg/kg. TAK-164 appeared to have a manageable safety profile up to 0.064 mg/kg in pts with advanced GI cancers.
|
||||
References
