Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0HVSBG
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
BDC-1001
|
|||||
| Synonyms |
BDC 1001; BDC-1001; Boltbody ISAC BDC-1001
Click to Show/Hide
|
|||||
| Organization |
Bolt Biotherapeutics, Inc.
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 5 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
Undisclosed
|
|||||
| Structure |
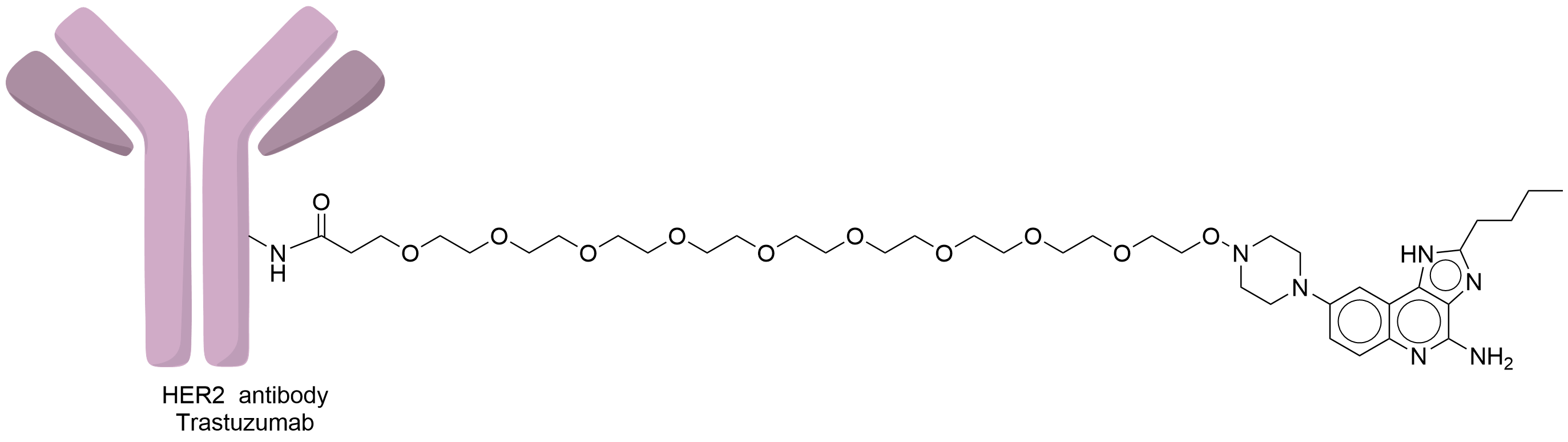
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
TORL7/8 agonist T785
|
Payload Info | ||||
| Therapeutic Target |
Toll-like receptor 7 (TLR7); Toll-like receptor 8 (TLR8)
|
Target Info | ||||
| Linker Name |
BG based linker
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
imbotolimod
|
|||||
| Puchem SID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
Patients with advanced metastatic HER2-expressing (IHC2/3+) or amplified solid tumors. Patients had received a median of 4 prior therapies.
|
||||
| Administration Dosage |
4 dose levels (0.15-5.00 mg/kg) every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04278144 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Phase 1/2 study of BDC-1001 as a single agent and in combination with nivolumab in patients with advanced HER2-expressing solid tumors. | ||||
References
