Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0HRDSA
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Anti-TNF ADC 121
|
|||||
| Synonyms |
Anti-TNF-ADC-121
Click to Show/Hide
|
|||||
| Organization |
AbbVie, Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
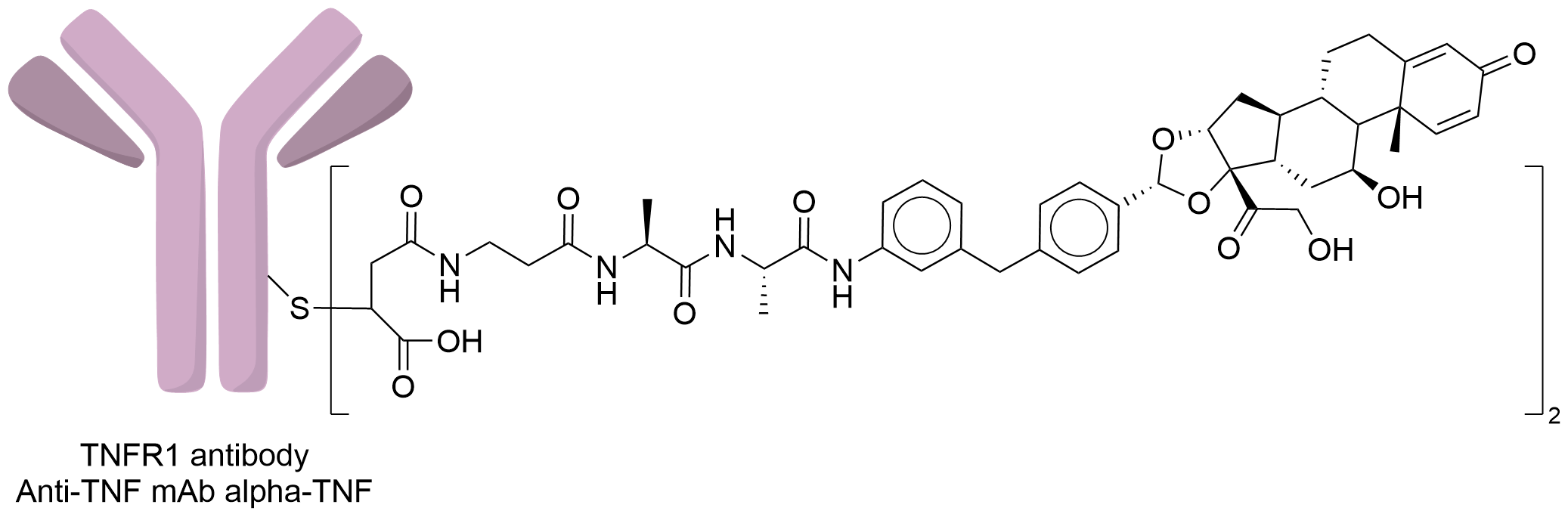
|
|||||
| Antibody Name |
Anti-TNF mAb alpha-TNF
|
Antibody Info | ||||
| Antigen Name |
Tumor necrosis factor receptor superfamily member 1A (TNFRSF1A)
|
Antigen Info | ||||
| Payload Name |
GRM cpd 17
|
Payload Info | ||||
| Therapeutic Target |
Glucocorticoid receptor (NR3C1)
|
Target Info | ||||
| Linker Name |
MP-Ala-Ala
|
Linker Info | ||||
| Conjugate Type |
The free sulfhydryl group obtained by the reduction of interchain cysteine is site-specific conjugated with maleimide, michael addition reaction, followed by incubation at basic ph to hydrolyze-open the maleimide ring.
|
|||||
General Information of The Activity Data Related to This ADC
Obtained from the Model Organism Data
| Standard Type | Value | Units | Animal Model (No. of PDX) |
|---|---|---|---|
| Paw swelling AUC |
74
|
%
|
Collagen-induced arthritis (mCIA) model
|
| Paw swelling AUC |
96
|
%
|
Collagen-induced arthritis (mCIA) model
|
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Paw swelling AUC | 74.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
To evaluate the impact of the anti-mTNF GRM ADCs on inflammation in a chronic inflammatory setting,we progressed several ADCs into a mouse collagen-induced arthritis (mCIA) model. A single 3 mg/kg dose of the ADC was given at the first clinical signs of disease,a time point of intervention where anti-TNF treatment has a moderate impact.
|
||||
| In Vivo Model | Collagen-induced arthritis (mCIA) model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Paw swelling AUC | 96.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
To evaluate the impact of the anti-mTNF GRM ADCs on inflammation in a chronic inflammatory setting,we progressed several ADCs into a mouse collagen-induced arthritis (mCIA) model. A single 10 mg/kg dose of the ADC was given at the first clinical signs of disease,a time point of intervention where anti-TNF treatment has a moderate impact.
|
||||
| In Vivo Model | Collagen-induced arthritis (mCIA) model | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.09 ug/mL | Positive TNF expression (TNF+++/++) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells (TNF expression) | CVCL_0004 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 43.70 ug/mL | Negative TNF expression (TNF-) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells | CVCL_0004 | ||
References
