Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0FHSSN
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
B7H3-EC3 DAR4.1
|
|||||
| Synonyms |
B7H3 EC3; B7H3EC3
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4.1
|
|||||
| Structure |
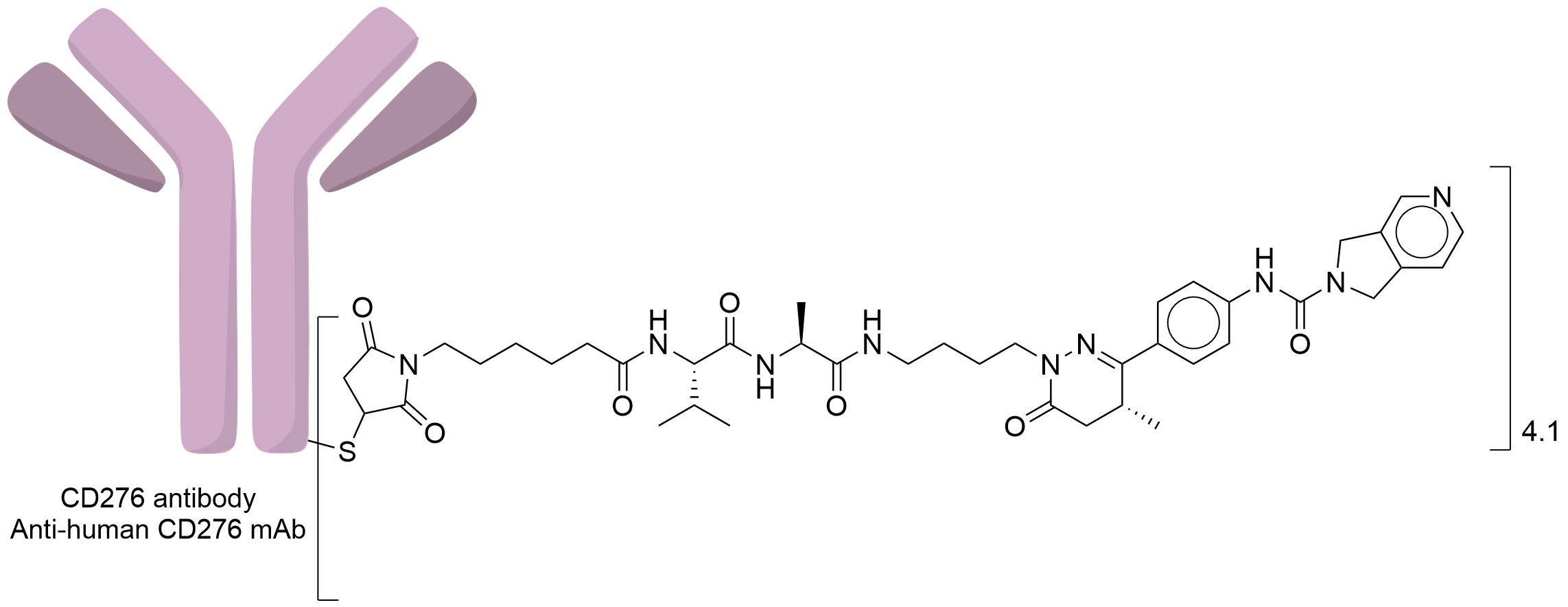
|
|||||
| Antibody Name |
Anti-human CD276 mAb
|
Antibody Info | ||||
| Antigen Name |
CD276 antigen (CD276)
|
Antigen Info | ||||
| Payload Name |
NAMPT inhibitor 4
|
Payload Info | ||||
| Therapeutic Target |
Nicotinamide phosphoribosyltransferase (NAMPT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Ala
|
Linker Info | ||||
| Conjugate Type |
Maleimide was used for Michael addition reactions with the thiol groups of the antibody that were generated through the reduction of interchain disulfide bonds.
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
≈ 90.9
|
%
|
THP-1 cells
|
Childhood acute monocytic leukemia
|
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.90% (Day 30) | High CD276 expression (CD276+++) | ||
| Method Description |
The in vivo antitumor efficacy and tolerability of the selected NAMPTi-ADCs were evaluated in the cell line-derived THP-1 human AmL xenograft model.Treatment with the low-DAR B7H3-EC3 (5 mg/kg,iv,Q7Dx3).
|
||||
| In Vivo Model | Monocytic leukemia CDX model | ||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.04 nM | High LYPD3 expression (LYPD3+++); High HER2 expression (HER2+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 uM | High CD276 expression (CD276+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 uM | High LYPD3 expression (LYPD3+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | A549-C4.4a cells | CVCL_0023 | ||
References
