Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0FBKIO
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
ABBV-3373
|
|||||
| Synonyms |
ABBV 3373; ABBV-3373; ABBV3773; anti-TNF-GRM-antibody-drug-conjugate; glucocorticoid receptor modulator (GRM) linked to adalimumab
Click to Show/Hide
|
|||||
| Organization |
AbbVie, Inc.
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
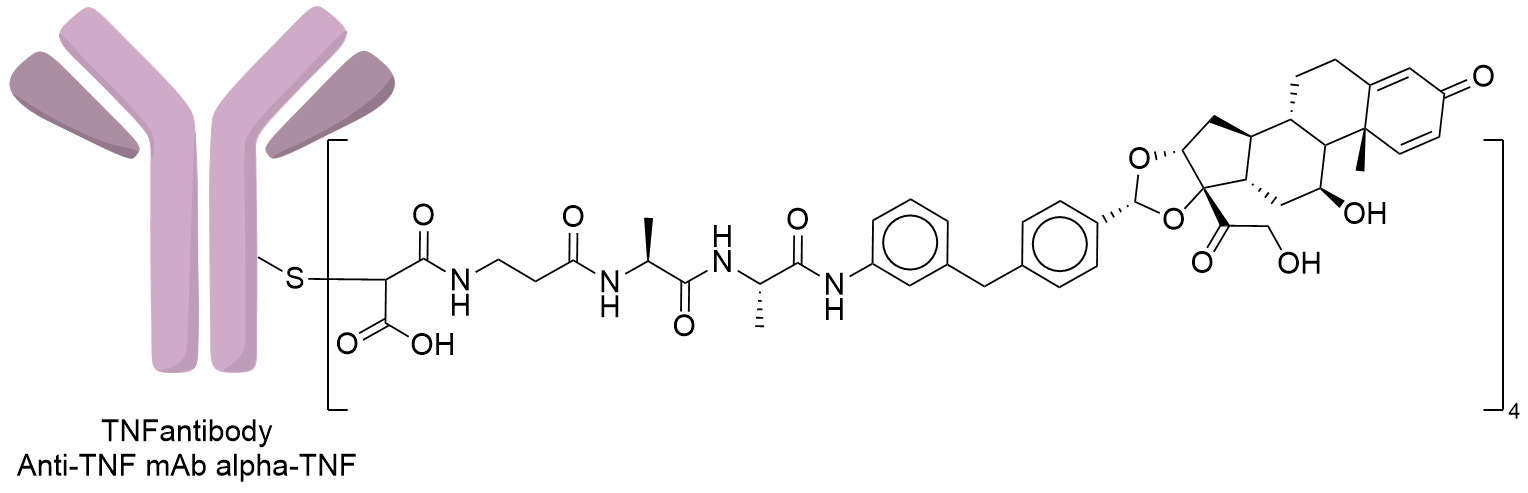
|
|||||
| Antibody Name |
Anti-TNF mAb alpha-TNF
|
Antibody Info | ||||
| Antigen Name |
Tumor necrosis factor receptor superfamily member 1A (TNFRSF1A)
|
Antigen Info | ||||
| Payload Name |
GRM cpd 17
|
Payload Info | ||||
| Therapeutic Target |
Glucocorticoid receptor (NR3C1)
|
Target Info | ||||
| Linker Name |
MP-Ala-Ala
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Obtained from the Model Organism Data
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
RA (based on the 1987 American College of Rheumatology [ACR] classification criteria or 2010 ACR/EULAR criteria [16, 17]), with disease duration >3 months, and active disease defined as a Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP) (18) of 3.2 and 4 of 28 swollen joints and 4 of 28 tender joints.
|
||||
| Administration Dosage |
Intravenously (IV) ABBV-3373 100 mg every other week for 12 weeks, followed by placebo for 12 weeks, or subcutaneous adalimumab 80 mg every other week for 24 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03823391 | Clinical Status | Phase 2 | ||
| Clinical Description | A randomized, double-blind, double-dummy, active controlled study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of ABBV-3373 in subjects with moderate to severe rheumatoid arthritis. | ||||
| Primary Endpoint |
48 patients were randomized to ABBV-3373 (n=31) or adalimumab (n=17). At week 12, ABBV-3373 reduced DAS28 (CRP) versus historical adalimumab (2.65 versus 2.13; P=0.022) and combined in-trial/historical adalimumab (2.65 versus 2.29; probability=89.9%), with numerically greater improvement than in-trial adalimumab (2.51).
|
||||
| Other Endpoint |
For secondary endpoints, greater efficacy was observed with ABBV-3373 versus historical adalimumab; ABBV-3373 was predicted with 79.30-99.50% probability to be better than adalimumab based on combined in-trial/historical adalimumab data.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03823391 | Clinical Status | Phase 2 | ||
| Clinical Description | A randomized, double-blind, double-dummy, active controlled study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of ABBV-3373 in subjects with moderate to severe rheumatoid arthritis. | ||||
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Paw swelling AUC | 81.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
To evaluate the impact of the anti-mTNF GRM ADCs on inflammation in a chronic inflammatory setting,we progressed several ADCs into a mouse collagen-induced arthritis (mCIA) model. A single 3 mg/kg dose of the ADC was given at the first clinical signs of disease,a time point of intervention where anti-TNF treatment has a moderate impact.
|
||||
| In Vivo Model | Collagen-induced arthritis (mCIA) model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Paw swelling AUC | 98.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
To evaluate the impact of the anti-mTNF GRM ADCs on inflammation in a chronic inflammatory setting,we progressed several ADCs into a mouse collagen-induced arthritis (mCIA) model. A single 10 mg/kg dose of the ADC was given at the first clinical signs of disease,a time point of intervention where anti-TNF treatment has a moderate impact.
|
||||
| In Vivo Model | Collagen-induced arthritis (mCIA) model | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | P1NP inhibition | 0.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess P1NP level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | P1NP inhibition | 19.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess P1NP level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Ear swelling inhibition | 55.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization.
|
||||
| In Vivo Model | Fluorescein isothiocyanate (FITC)-induced CHS model | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Ear swelling inhibition | 88.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization.
|
||||
| In Vivo Model | Fluorescein isothiocyanate (FITC)-induced CHS model | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Corticosterone inhibition | 6.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess corticosterone level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Corticosterone inhibition | 15.00% | Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess corticosterone level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.08 ug/mL | Positive TNF expression (TNF+++/++) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells (TNF expression) | CVCL_0004 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 5.80 ug/mL | Negative TNF expression (TNF-) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells | CVCL_0004 | ||
References
