Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0BIQVN
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
AZD5335
|
|||||
| Synonyms |
AZD 5335; AZD-5335; AZD5335
Click to Show/Hide
|
|||||
| Organization |
AstraZeneca PLC
|
|||||
| Drug Status |
Phase 1/2
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
8
|
|||||
| Structure |
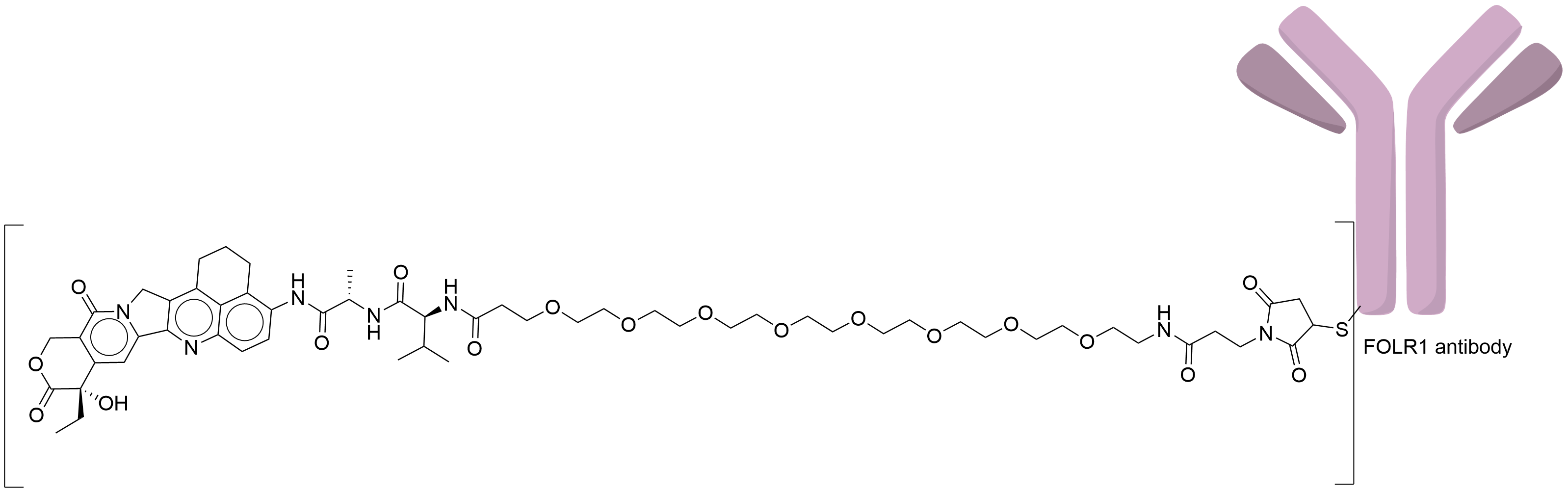
|
|||||
| Antibody Name |
Undisclosed
|
|||||
| Antigen Name |
Folate receptor alpha (FOLR1)
|
Antigen Info | ||||
| Payload Name |
AZ14170132
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Mal-PEG8-Val-Ala
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
samrotecan
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05797168 | Clinical Status | Phase 1/2 | ||
| Clinical Description | FONTANA: A modular phase 1/2a, open-label, multi-center study to assess the safety, tolerability, pharmacokinetics, and preliminary efficacy of ascending doses of AZD5335 monotherapy and in combination with anti-cancer agents in participants with solid tumors. | ||||
| Primary Endpoint |
Number of participants with adverse events/serious adverse events, the number of participants with dose limiting toxicity (DLT).
|
||||
| Other Endpoint |
Objective Response Rate (ORR), Duration of Response (DoR), Disease Control Rate (DCR), Progression free Survival (PFS), Overall Survival (OS).
|
||||
References
