Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ARIWN
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
CMD-193
|
|||||
| Synonyms |
CD193; CMD 193; CMD-193; Hu3S193-CM; Hu3S193-CalichDMH
Click to Show/Hide
|
|||||
| Organization |
Wyeth AB; Pfizer Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
Undisclosed
|
|||||
| Structure |
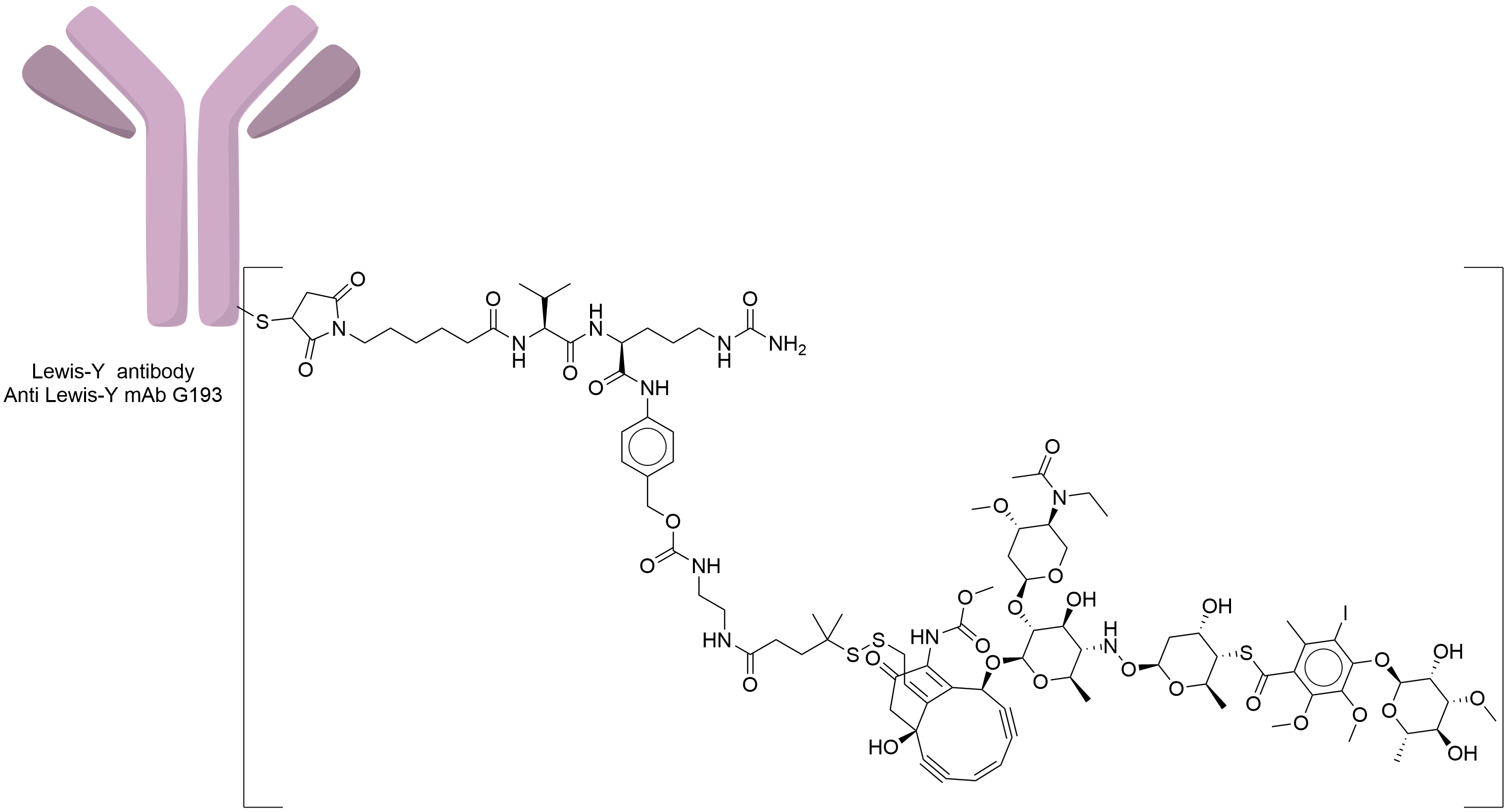
|
|||||
| Antibody Name |
Anti-Lewis-Y mAb G193
|
Antibody Info | ||||
| Antigen Name |
Lewis Y
|
Antigen Info | ||||
| Payload Name |
N-acetyl-gamma-calicheamicin
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
AcButDMH
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
≈ 99
|
%
|
NCI-N87 cells
|
Gastric tubular adenocarcinoma
|
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Complete Remission (CR) |
0.00%
|
|||
| Patients Enrolled |
Patients with advanced tumours expressing the Lewis-Y antigen.
|
||||
| Administration Dosage |
111-In-CMD-193 iv then 1.00 mg/m^2, 2.60 mg/m^2 iv of CMD-193.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00293215 | Clinical Status | Phase 1 | ||
| Clinical Description | Biodistribution study of cmd-193 in patients with advanced tumours expressing the LEWIS-Y antigen. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Patients Enrolled |
Patients with advanced malignant tumors.
|
||||
| Administration Dosage |
CMD-193 iv.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00257881 | Clinical Status | Phase 1 | ||
| Clinical Description | Phase 1 biodistribution study of 111-indium-CMD-193 in patients with advanced tumours expressing the LEWIS-Y antigen. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Patients Enrolled |
Patients with advanced malignant tumors.
|
||||
| Administration Dosage |
CMD-193 iv.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00161642 | Clinical Status | Phase 1 | ||
| Clinical Description | Phase 1 dose-escalation study of intravenous CMD-193 in subjects with advanced malignant solid tumors. | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.00% (Day 100) | Positive Lewis-Y expression (Lewis-Y +++/++) | ||
| Method Description |
hu3S193-CalichDMH (4 ug) induces efficient tumor cell killing in cell line-derived models of PV-1 cells with CD28 expression.
|
||||
| In Vivo Model | N193 CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
References
