Payload Information
General Information of This Payload
| Payload ID | PAY0VZJSJ |
|||||
|---|---|---|---|---|---|---|
| Name | seco-CBI-indole |
|||||
| Synonyms |
SECO-CBI-INDOLE; CHEMBL1276856; SCHEMBL12596993; 1alpha-(Chloromethyl)-2,3-dihydro-3-[[5-[[(1H-indole-2-yl)carbonyl]amino]-1H-indole-2-yl]carbonyl]-1H-benzo[e]indole-5-ol
Click to Show/Hide
|
|||||
| Target(s) | Human Deoxyribonucleic acid (hDNA) | |||||
| Structure |
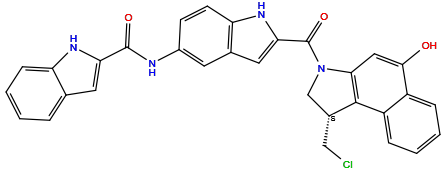
|
|||||
| Formula | C31H23ClN4O3 |
|||||
| Isosmiles | C1[C@H](C2=C(N1C(=O)C3=CC4=C(N3)C=CC(=C4)NC(=O)C5=CC6=CC=CC=C6N5)C=C(C7=CC=CC=C72)O)CCl |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C31H23ClN4O3/c32-15-19-16-36(27-14-28(37)21-6-2-3-7-22(21)29(19)27)31(39)26-13-18-11-20(9-10-24(18)35-26)33-30(38)25-12-17-5-1-4-8-23(17)34-25/h1-14,19,34-35,37H,15-16H2,(H,33,38)/t19-/m1/s1
|
|||||
| InChIKey |
OMTPLEBPJORDFA-LJQANCHMSA-N
|
|||||
| IUPAC Name |
N-[2-[(1S)-1-(chloromethyl)-5-hydroxy-1,2-dihydrobenzo[e]indole-3-carbonyl]-1H-indol-5-yl]-1H-indole-2-carboxamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
535 |
Polar area |
101 |
||
Complexity |
936 |
xlogp Value |
6 |
|||
Heavy Count |
39 |
Rot Bonds |
4 |
|||
Hbond acc |
3 |
Hbond Donor |
4 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
OHPAS ADC-3 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 78) | High HER2 expression (HER2 +++) | ||
| Method Description |
We also tested the OHPAS ADCs in in vivo xenograft mouse models using N87 cell lines. The experiment was followed up to 110 days after administration of ADCs at two different doses (0.5 mg/kg) on day one (initial tumor volume 100 mm3).
|
||||
| In Vivo Model | NCI-N87 CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.10% (Day 78) | High HER2 expression (HER2 +++) | ||
| Method Description |
We also tested the OHPAS ADCs in in vivo xenograft mouse models using N87 cell lines. The experiment was followed up to 110 days after administration of ADCs at two different doses (2 mg/kg) on day one (initial tumor volume 100 mm3).
|
||||
| In Vivo Model | NCI-N87 CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
Moderate HER2 expression (HER2 ++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.58 nM
|
Negative HER2 expression (HER2 -) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
References
