Payload Information
General Information of This Payload
| Payload ID | PAY0VYKOO |
|||||
|---|---|---|---|---|---|---|
| Name | Tacrolimus |
|||||
| Synonyms |
Tacrolimus; Fujimycin; 104987-11-3; Prograf; Protopic; Tsukubaenolide; Tacrolimus anhydrous; FK506; Advagraf; Modigraf; Anhydrous Tacrolimus; Prograft; Fk-506; Protopy; LCP-Tacro; FK 506; Avagraf; Envarsus; FR-900506; tacrolimus (fk506); Astagraf XL; Envarsus XR; Tacrolimus, anhydrous; Tacrolimus (anhydrous); Graceptor; Hecoria; (-)-FK 506; 8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN; Tacrolimus (INN); Prograf (TN); UNII-Y5L2157C4J; PROGRAPH; TACRO; CCRIS 7124; DTXSID5046354; FR900506; K506; CHEBI:61049; HSDB 8195; FR 900506; Tacrolimus hydrate; Y5L2157C4J; Tacrolimus [USAN:INN]; FK-506 (Tacrolimus); Tacrolimus [USAN]; NSC-758659; L 679934; Tacarolimus; CHEMBL269732; DTXCID3026354; NCGC00163470-03; Protopic (TN); TACROLIMUS [INN]; TACROLIMUS (MART.); TACROLIMUS [MART.]; (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-Hexadecahydro-5,19-dihydroxy-3-[(1E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propen-1-yl)-15,19-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)tetrone; L-679934; TACROLIMUS (USP-RS); FK5; CHEBI:61057; C44H69NO12.H2O; TACROLIMUS (USP MONOGRAPH); 8-DEETHYL-8-(BUT-3-ENYL)-ASCOMYCIN; NSC 758659; SR-05000001879; TACROLIMUS MONOHYDRATE (EP MONOGRAPH); tacrolimusum; Talymus; TacroBell; MFCD00869853; NSC717865; Advagraf (TN); Tacrolimus (Prograf); ENVARSUS-XR; TACROLIMUS [MI]; D06OMK; SCHEMBL3088; TACROLIMUS [WHO-DD]; BSPBio_001279; CHEMBL66247; Tacrolimus [USAN:BAN:INN]; GTPL6784; CHEBI:93221; HMS503O21; D11AH01; L04AD02; QJJXYPPXXYFBGM-LFZNUXCKSA-N; HMS1792O21; HMS1990O21; HMS2093M19; HMS3403O21; Pharmakon1600-01503968; 15,19-Epoxy-3H-pyrido(2,1-c)(1,4)oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-(2-(4-hydroxy-3-methoxycyclohexyl)-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-, (3S-(3R*(E(1S*,3S*,4S*)),4S*,5R*,8S*,9E,12R*,14R*,15S*,16R*,18S*,19S*,26aR*))-; EX-A1677; Tox21_112056; BDBM50030448; BDBM50079777; HB0289; LMPK04000003; NSC758659; s5003; AKOS005145901; AC-1182; AM81227; CCG-270494; CS-1507; DB00864; IDI1_001040; NCGC00163470-01; NCGC00163470-02; NCGC00163470-04; NCGC00163470-05; NCGC00163470-06; NCGC00163470-07; NCGC00163470-27; HY-13756; SBI-0052894.P002; CAS-104987-11-3; M2258; C01375; D08556; EN300-221601; AB01209746-01; AB01209746_03; Q411648; Q-201775; SR-05000001879-1; SR-05000001879-2; SR-05000001879-5; BRD-K35452788-001-02-1; BRD-K69608737-001-03-7; BRD-K69608737-001-10-2; Z2242006187; [(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethenyl]-14,16-; 15,19-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(23H)-tetrone; 15,19-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(23H)-tetrone,; (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-1,14-dihydroxy-12-[(E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-23,25-dimethoxy-13,19,21,27-tetramethyl-17-prop-2-enyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetrone; (1R,9S,12S,13R,14S,17R,21S,23S,24R,25S,27R)-1,14-dihydroxy-12-{1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl}-23,25-dimethoxy-13,19,21,27-tetramethyl-17-(prop-2-en-1-yl)-11,28-dioxa-4-azatricyclo[22.3.1.0,4,9]octacos-18-ene-2,3,10,16-tetrone; (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-5,19-dihydroxy-3-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl}-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(prop-2-en-1-yl)-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-3H-15,19-epoxypyrido[2,1-c][1,4]oxazacyclotricosine-1,7,20,21(4H,23H)-tetrone; (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26AS)-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26A-HEXADECAHYDRO-5,19-DIHYDROXY-3-[(1E)-2-[(1R,3R,4R)-4-HYDROXY-3-METHOXYCYCLOHEXYL]-1-METHYLETHENYL]-14,16-DIMETHOXY-4,10,12,18-TETRAMETHYL-8-(2-PROPEN-1-YL)-15,19; (E)-(1R,9S,12S,13R,14R,21S,23S,24R,25S,27R)-17-Allyl-1,14-dihydroxy-12-[(E)-2-((3R,4R)-4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-vinyl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[22.3.1.0*4,9*]octacos-18-ene-2,3,10,16-tetraone; 15 19-Epoxy-3H-pyrido[2 1-c][1 4]oxaazacyclotricosine-1 7 20 21(4H 23H)-tetrone 5 6 8 11 12 13 14 15 16 17 18 19 24 25 26 26a-hexadecahydro-5 19-dihydroxy-3-[(1E)-2-[(1R 3R 4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethenyl]-14 16-dimethoxy-4 10 12 18-tet; 15 19-Epoxy-3H-pyrido[2 1-c][1 4]oxaazacyclotricosine-1 7 20 21(4H 23H)-tetrone 5 6 8 11 12 13 14 15 16 17 18 19 24 25 26 26a-hexadecahydro-5 19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14 16-dimethoxy-4 10 12 18-tetramethyl-8-(2-pr; 15,19-epoxi-3h-pirido[2,1-c][1,4]oxaazaciclotricosina-1,7,20,21(4h,23h)-tetrona, 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahidro-5,19-dihidroxi-3-[(1e)-2-[(1r,3r,4r)-4-hidroxi-3-metoxiciclohexil]-1-metiletenil]-14,16-dimetoxi-4,10,12,18-tetram; 15,19-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[(1E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyc; 15,19-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[(1E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propen-1-yl)-, (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-; 15,19-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycycl ohexyl]-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propen-1-yl)-, (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-; 4,5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-heptadecahydro-5,19-dihydroxy-3-; dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-,(3S,4R,5S,8R,12S,14S,15R,16S,18R,19R,26aS)-; lohexyl]-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propen-1-yl)-, (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-
Click to Show/Hide
|
|||||
| Target(s) | Peptidyl-prolyl cis-trans isomerase FKBP1A (FKBP1A) | |||||
| Structure |
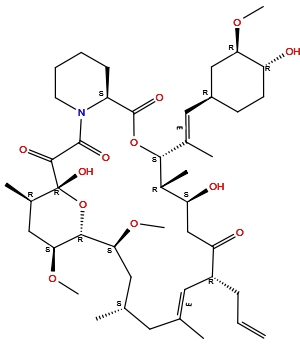
|
|||||
| Formula | C44H69NO12 |
|||||
| Isosmiles | C[C@@H]1C[C@@H]([C@@H]2[C@H](C[C@H]([C@@](O2)(C(=O)C(=O)N3CCCC[C@H]3C(=O)O[C@@H]([C@@H]([C@H](CC(=O)[C@@H](/C=C(/C1)\C)CC=C)O)C)/C(=C/[C@@H]4CC[C@H]([C@@H](C4)OC)O)/C)O)C)OC)OC |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1
|
|||||
| InChIKey |
QJJXYPPXXYFBGM-LFZNUXCKSA-N
|
|||||
| IUPAC Name |
(1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-1,14-dihydroxy-12-[(E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-23,25-dimethoxy-13,19,21,27-tetramethyl-17-prop-2-enyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetrone
|
|||||
| Pharmaceutical Properties | Molecule Weight |
804 |
Polar area |
178 |
||
Complexity |
1480 |
xlogp Value |
2.7 |
|||
Heavy Count |
57 |
Rot Bonds |
7 |
|||
Hbond acc |
12 |
Hbond Donor |
3 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.000015 | ug/mL |
T-cells
|
Normal
|
Undisclosed | [1] |
| Half Maximal Inhibitory Concentration (IC50) | 0.000015 | ug/mL |
T-cells
|
Normal
|
Undisclosed | [2] |
| Half Maximal Inhibitory Concentration (IC50) | 0.0016 | ug/mL |
B-cells
|
Normal
|
Undisclosed | [1] |
| Half Maximal Inhibitory Concentration (IC50) | 0.034 | nM |
CD4+ve Th cells
|
Normal
|
Undisclosed | [3] |
| Half Maximal Inhibitory Concentration (IC50) | 0.18 | nM |
T-cells
|
Normal
|
Undisclosed | |
| Half Maximal Inhibitory Concentration (IC50) | 0.19 | nM |
Jurkat cells
|
T acute lymphoblastic leukemia
|
[4] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.25 | nM |
RBL-2H3 cells
|
Rat leukemia
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.25 | nM |
RBL-2H3 cells
|
Rat leukemia
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.25 | nM |
RBL-2H3 cells
|
Rat leukemia
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.29 | nM |
T-cells
|
Normal
|
Undisclosed | |
| Half Maximal Inhibitory Concentration (IC50) | 0.29 | nM |
T-cells
|
Normal
|
Undisclosed | |
| Half Maximal Inhibitory Concentration (IC50) | 0.29 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 0.5 | nM |
T-cells
|
Normal
|
Undisclosed | [8] |
| Half Maximal Inhibitory Concentration (IC50) | 0.9 | nM |
T-cells
|
Normal
|
Undisclosed | [8] |
| Half Maximal Inhibitory Concentration (IC50) | 10.9 | nM |
WiDr cells
|
Colon adenocarcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | >100000 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | >100000 | nM |
Vero 76 cells
|
Normal
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 10495.93 | nM |
D283 Med cells
|
Medulloblastoma
|
||
| Half Maximal Inhibitory Concentration (IC50) | 13.8 | nM |
U-251MG cells
|
Astrocytoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 2 | nM |
Jurkat cells
|
T acute lymphoblastic leukemia
|
[11] | |
| Half Maximal Inhibitory Concentration (IC50) | 3300 | nM |
HEK293 cells
|
Normal
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 360 | nM |
Jurkat cells
|
T acute lymphoblastic leukemia
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 3700 | nM |
HEK293 cells
|
Normal
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 38290 | nM |
HEK293 cells
|
Normal
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 5800 | nM |
Jurkat cells
|
T acute lymphoblastic leukemia
|
[15] |
Each Antibody-drug Conjugate Related to This Payload
References
