Payload Information
General Information of This Payload
| Payload ID | PAY0USVCB |
|||||
|---|---|---|---|---|---|---|
| Name | Duostatin 5.2 |
|||||
| Synonyms |
Duostatin 5.2
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
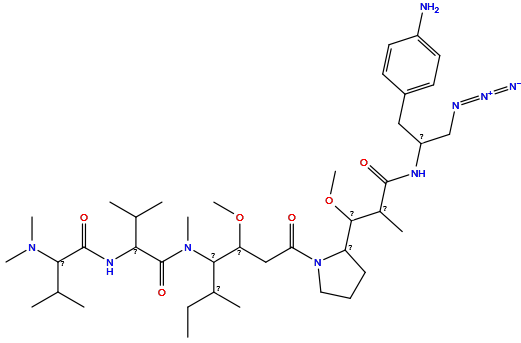
|
|||||
| Formula | C40H69N9O6 |
|||||
| Isosmiles | CCC(C)C(C(CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(CN=[N+]=[N-])Cc1ccc(N)cc1)OC)N(C)C(=O)C(NC(=O)C(C(C)C)N(C)C)C(C)C |
|||||
| InChI |
InChI=1S/C40H69N9O6/c1-13-26(6)36(48(10)40(53)34(24(2)3)45-39(52)35(25(4)5)47(8)9)32(54-11)22-33(50)49-20-14-15-31(49)37(55-12)27(7)38(51)44-30(23-43-46-42)21-28-16-18-29(41)19-17-28/h16-19,24-27,30-32,34-37H,13-15,20-23,41H2,1-12H3,(H,44,51)(H,45,52)
|
|||||
| InChIKey |
QIHHTRNUHMRKQK-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
772.049 |
Polar area |
195.3 |
||
Complexity |
55 |
xlogp Value |
4.2537 |
|||
Heavy Count |
55 |
Rot Bonds |
22 |
|||
Hbond acc |
9 |
Hbond Donor |
3 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
STI-6129 [Phase 1/2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
Patients with relapsed/refractory (RR) myeloma.
|
||||
| Administration Dosage |
Dose range from 0.25 mg/kg to 3.68 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05565807 | Phase Status | Phase 1/2 | ||
| Clinical Description |
A phase 1b/2a, open-label, dose-escalation and extension study to evaluate the safety and efficacy of an anti-CD38 antibody drug conjugate (STI-6129) in patients with relapsed or refractory multiple myeloma.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05308225 | Phase Status | Phase 1/2 | ||
| Clinical Description |
A phase 1b/2a, open-label, dose-escalation study of the safety and efficacy of an anti-CD38 antibody drug conjugate (STI-6129) in patients with relapsed or refractory multiple myeloma.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04316442 | Phase Status | Phase 1/2 | ||
| Clinical Description |
A phase 1b/2a, open-label, dose-escalation study of the safety and efficacy of an anti-CD38 antibody drug conjugate (STI-6129) in patients with relapsed or refractory systemic al amyloidosis.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05692908 | Phase Status | Phase 1 | ||
| Clinical Description |
An open-label, dose-escalation study of the safety of an anti-CD38 antibody drug conjugate (STI-6129) in patients with relapsed or refractory systemic al amyloidosis.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05584709 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1b, open-label, dose-escalation study of the safety and efficacy of an anti-CD38 antibody drug conjugate (STI-6129) in patients with advanced solid tumors.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05519527 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 study of safety and efficacy of an anti-CD38 antibody drug conjugate (STI-6129) in patients with relapsed or refractory t-acute lymphoblastic leukemia/lymphoma (T-ALL) or acute myeloid leukemia (AML).
|
||||
References
