Payload Information
General Information of This Payload
| Payload ID | PAY0URNSM |
|||||
|---|---|---|---|---|---|---|
| Name | Cryptophycin |
|||||
| Synonyms |
Cryptophycin 1; cryptophycin; 124689-65-2; UNII-XO974V2M5T; C35H43ClN2O8; XO974V2M5T; (3S,6R,10R,13E,16S)-10-[(3-chloro-4-methoxyphenyl)methyl]-6-methyl-3-(2-methylpropyl)-16-[(1S)-1-[(2R,3R)-3-phenyloxiran-2-yl]ethyl]-1,4-dioxa-8,11-diazacyclohexadec-13-ene-2,5,9,12-tetrone; CRYPTOPHYCIN A; 10-((3-Chloro-4-methoxyphenyl)methyl)-6-methyl-3-(2-methylpropyl)-16-(1-(3-phenyloxiranyl)ethyl)-1,4-dioxa-8,11-diazacyclohexadec-13-ene-2,5,9,12-tetrone; 1,4-Dioxa-8,11-diazacyclohexadec-13-ene-2,5,9,12-tetrone, 10-((3-chloro-4-methoxyphenyl)methyl)-6-methyl-3-(2-methylpropyl)-16-(1-(3-phenyloxiranyl)ethyl)-; CRYPTOPHYCIN-1; D0LI3C; CRYPTOPHYCIN-1 [MI]; CHEMBL441018; SCHEMBL1346966; PSNOPSMXOBPNNV-VVCTWANISA-N; DTXSID901046187; C35-H43-Cl-N2-O8; LS-172597; C16877; Q27293933; .BETA.-ALANINE, N-(3-CHLORO-N-(5-((2-HYDROXY-4-METHYL-1-OXOPENTYL)OXY)-1-OXO-6-(3-PHENYLOXIRANYL)-2-HEPTENYL)-O-METHYL-D-TYROSYL)-2-METHYL-, .XI.-LACTONE, (2R-(2.ALPHA.(1(R*),2E,5S*(S*),6S*),3.BETA.))-; CYCLO((2R)-2-METHYL-.BETA.-ALANYL-(2S)-2-HYDROXY-4-METHYLPENTANOYL-(2E,5S,6S)-5-HYDROXY-6-((2R,3R)-3-PHENYL-2-OXIRANYL)-2-HEPTENOYL-3-CHLORO-O-METHYL-D-TYROSYL); CYCLO((2R)-2-METHYL-.BETA.-ALANYL-(2S)-2-HYDROXY-4-METHYLPENTANOYL-(2E,5S,6S)-5-HYDROXY-6-((2R,3R)-3-PHENYLOXIRANYL)-2-HEPTENOYL-3-CHLORO-O-METHYL-D-TYROSYL)
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
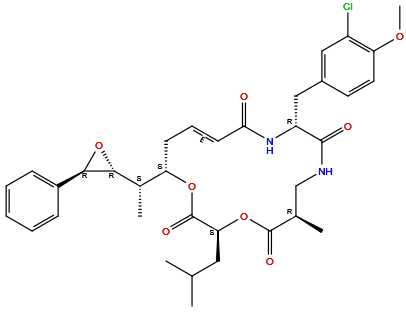
|
|||||
| Formula | C35H43ClN2O8 |
|||||
| Isosmiles | C[C@@H]1CNC(=O)[C@H](NC(=O)/C=C/C[C@H](OC(=O)[C@@H](OC1=O)CC(C)C)[C@H](C)[C@@H]2[C@H](O2)C3=CC=CC=C3)CC4=CC(=C(C=C4)OC)Cl |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C35H43ClN2O8/c1-20(2)16-29-35(42)44-27(22(4)31-32(46-31)24-10-7-6-8-11-24)12-9-13-30(39)38-26(33(40)37-19-21(3)34(41)45-29)18-23-14-15-28(43-5)25(36)17-23/h6-11,13-15,17,20-22,26-27,29,31-32H,12,16,18-19H2,1-5H3,(H,37,40)(H,38,39)/b13-9+/t21-,22+,26-,27+,29+,31-,32-/m1/s1
|
|||||
| InChIKey |
PSNOPSMXOBPNNV-VVCTWANISA-N
|
|||||
| IUPAC Name |
(3S,6R,10R,13E,16S)-10-[(3-chloro-4-methoxyphenyl)methyl]-6-methyl-3-(2-methylpropyl)-16-[(1S)-1-[(2R,3R)-3-phenyloxiran-2-yl]ethyl]-1,4-dioxa-8,11-diazacyclohexadec-13-ene-2,5,9,12-tetrone
|
|||||
| Pharmaceutical Properties | Molecule Weight |
655.2 |
Polar area |
133 |
||
Complexity |
1080 |
xlogp Value |
6.2 |
|||
Heavy Count |
46 |
Rot Bonds |
8 |
|||
Hbond acc |
8 |
Hbond Donor |
2 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
FOLR1-Mal-PEG-Val-Cit-PABC-Cryptophycin [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
High FOLR1 expression(FOLR1+++) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Moderate FOLR1 expression(FOLR1++) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Lung non-small cell carcinoma | NCI-H2110 cells | CVCL_1530 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Low FOLR1 expression(FOLR1+) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative FOLR1 expression(FOLR1-) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Osteosarcoma | SJSA-1 cells | CVCL_1697 | ||
References
