Payload Information
General Information of This Payload
| Payload ID | PAY0UIZBY |
|||||
|---|---|---|---|---|---|---|
| Name | Monomethyl auristatin D |
|||||
| Synonyms |
MMAD; 203849-91-6; Monomethylauristatin D; Monomethyl Dolastatin 10; Demethyldolastatin 10; (S)-N-((3R,4S,5S)-3-methoxy-1-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)pyrrolidin-1-yl)-5-methyl-1-oxoheptan-4-yl)-N,3-dimethyl-2-((S)-3-methyl-2-(methylamino)butanamido)butanamide; (2S)-N-[(2S)-1-[[(3R,4S,5S)-3-methoxy-1-[(2S)-2-[(1R,2R)-1-methoxy-2-methyl-3-oxo-3-[[(1S)-2-phenyl-1-(1,3-thiazol-2-yl)ethyl]amino]propyl]pyrrolidin-1-yl]-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]-3-methyl-2-(methylamino)butanamide; L-Valinamide, N-methyl-L-valyl-N-[(1S,2R)-2-methoxy-4-[(2S)-2-[(1R,2R)-1-methoxy-2-methyl-3-oxo-3-[[(1S)-2-phenyl-1-(2-thiazolyl)ethyl]amino]propyl]-1-pyrrolidinyl]-1-[(1S)-1-methylpropyl]-4-oxobutyl]-N-methyl-; L-VALINAMIDE, N-METHYL-L-VALYL-N-((1S,2R)-2-METHOXY-4-((2S)-2-((1R,2R)-1-METHOXY-2-METHYL-3-OXO-3-(((1S)-2-PHENYL-1-(2-THIAZOLYL)ETHYL)AMINO)PROPYL)-1-PYRROLIDINYL)-1-((1S)-1-METHYLPROPYL)-4-OXOBUTYL)-N-METHYL-; W4ZIA40FZ9; MONOMETHYLDOLASTATIN 10; SCHEMBL1687441; CHEMBL3359823; DTXSID101010182; AMY30772; EX-A2650; AKOS032960443; CS-1613; BS-15896; HY-15581; S6765; D83666; A926322; N-METHYL-L-VALYL-N-((1S,2R)-2-METHOXY-4-((2S)-2-((1R,2R)-1-METHOXY-2-METHYL-3-OXO-3-(((1S)-2-PHENYL-1-(2-THIAZOLYL)ETHYL)AMINO)PROPYL)-1-PYRROLIDINYL)-1-((1S)-1-METHYLPROPYL)-4-OXOBUTYL)-N-METHYL-L-VALINAMIDE
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
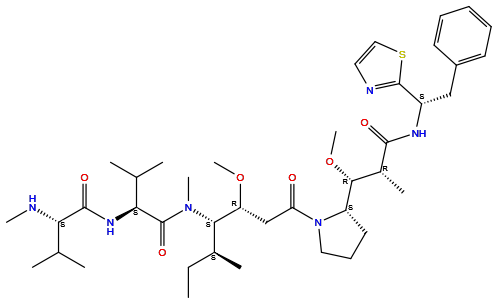
|
|||||
| Formula | C41H66N6O6S |
|||||
| Isosmiles | CC[C@H](C)[C@@H]([C@@H](CC(=O)N1CCC[C@H]1[C@@H]([C@@H](C)C(=O)N[C@@H](CC2=CC=CC=C2)C3=NC=CS3)OC)OC)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](C(C)C)NC |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C41H66N6O6S/c1-12-27(6)36(46(9)41(51)35(26(4)5)45-39(50)34(42-8)25(2)3)32(52-10)24-33(48)47-21-16-19-31(47)37(53-11)28(7)38(49)44-30(40-43-20-22-54-40)23-29-17-14-13-15-18-29/h13-15,17-18,20,22,25-28,30-32,34-37,42H,12,16,19,21,23-24H2,1-11H3,(H,44,49)(H,45,50)/t27-,28+,30-,31-,32+,34-,35-,36-,37+/m0/s1
|
|||||
| InChIKey |
BLUGYPPOFIHFJS-UUFHNPECSA-N
|
|||||
| IUPAC Name |
(2S)-N-[(2S)-1-[[(3R,4S,5S)-3-methoxy-1-[(2S)-2-[(1R,2R)-1-methoxy-2-methyl-3-oxo-3-[[(1S)-2-phenyl-1-(1,3-thiazol-2-yl)ethyl]amino]propyl]pyrrolidin-1-yl]-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]-3-methyl-2-(methylamino)butanamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
771.1 |
Polar area |
170 |
||
Complexity |
1190 |
xlogp Value |
5.3 |
|||
Heavy Count |
54 |
Rot Bonds |
21 |
|||
Hbond acc |
9 |
Hbond Donor |
3 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
RC88-PY-MAA-Val-Cit-PAB-MMAD [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 23.80% (Day 21) | High MSLN expression (MSLN+++/++) | ||
| Method Description |
Inoculate mice with gastric cancer cells at 3 million cells/mouse suspended in HBSS/matrigel, in the thoracic mammary fat pad at a volume of 0.2 ml. When tumors have reached a mean tumor volume of 100-250 mm3, they will be grouped. A single treatment will be administered intravenously (2 mg/kg, qwx3) via the tail vein on Day 0.
|
||||
| In Vivo Model | Oval-CitAR-3 CDX model | ||||
| In Vitro Model | Ovarian cancer | Oval-CitAR-3 cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
High MSLN expression (MSLN+++/++) | ||
| Method Description |
Cell-based in vitro assays are used to measure viability (proliferation), cytotoxicity,and induction of apoptosis of the ADC of the invention. Culturing the cells for a period from about 6 hours to about 5 days and measuring cell viability.
|
||||
| In Vitro Model | Ovarian cancer | Oval-CitAR-3 cells | Homo sapiens | ||
Zapadcine-1 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 14) | Positive DR5 expression (DR5+++/++) | ||
| Method Description |
Zapadcine-1 (9 mg/kg, Q3D3, every three days for 3 times) induces efficient tumor cell killing in cell line-derived models of Jurkat E6-1 cells with DR5 expression with high expression.
|
||||
| In Vivo Model | Jurkat E6.1 CDX model | ||||
| In Vitro Model | Leukemia | Jurkat E6.1 cells | CVCL_0367 | ||
RC88-Mc-Val-Cit-PAB-MMAD [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 21) | High MSLN expression (MSLN+++/++) | ||
| Method Description |
Inoculate mice with gastric cancer cells at 3 million cells/mouse suspended in HBSS/matrigel, in the thoracic mammary fat pad at a volume of 0.2 ml. When tumors have reached a mean tumor volume of 100-250 mm3, they will be grouped. A single treatment will be administered intravenously (2 mg/kg, qwx3) via the tail vein on Day 0.
|
||||
| In Vivo Model | Oval-CitAR-3 CDX model | ||||
| In Vitro Model | Ovarian cancer | Oval-CitAR-3 cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
High MSLN expression (MSLN+++/++) | ||
| Method Description |
Cell-based in vitro assays are used to measure viability (proliferation), cytotoxicity,and induction of apoptosis of the ADC of the invention. Culturing the cells for a period from about 6 hours to about 5 days and measuring cell viability.
|
||||
| In Vitro Model | Ovarian cancer | Oval-CitAR-3 cells | Homo sapiens | ||
12A11-TG6-vcMMAD [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.87 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
3G10-TG6-vcMMAD [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.90 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
6B6-TG6-vcMMAD [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.90 nM
|
Positive CXCR4 expression (CXCR4+++/++) | ||
| Method Description |
Cells were incubated with increasing concentrations of each ADCs at 37°C for 6 days in complete culture medium.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
References
