Payload Information
General Information of This Payload
| Payload ID | PAY0SVMRF |
|||||
|---|---|---|---|---|---|---|
| Name | Zoledronic acid |
|||||
| Synonyms |
Zoledronic acid; 118072-93-8; Zoledronate; Zometa; Reclast; Aclasta; (1-Hydroxy-2-(1H-imidazol-1-yl)ethane-1,1-diyl)diphosphonic acid; CGP 42446; (1-Hydroxy-2-imidazol-1-ylethylidene)diphosphonic acid; Zoledronic Acid Anhydrous; Orazol; C5H10N2O7P2; Anhydrous Zoledronic Acid; (1-hydroxy-2-imidazol-1-yl-1-phosphonoethyl)phosphonic acid; Bisphosphonate 3; Phosphonic acid, [1-hydroxy-2-(1H-imidazol-1-yl)ethylidene]bis-; ZOL; CGP-42446; Zometa (Novartis); Reclast (TN); Zoledronic acid [USAN:INN]; Zoledronic-d3 Acid; Zometa (TN); Zoledronic Acid Teva; Zoledronic Acid Medac; ZOL 446; Zoledronic acid (INN); CHEMBL924; Zoledronic Acid, Anhydrous; NSC-721517; [1-hydroxy-2-(1H-imidazol-1-yl)ethane-1,1-diyl]bis(phosphonic acid); UNII-70HZ18PH24; 2-(imidazol-1-yl)-1-hydroxyethane-1,1-diphosphonic acid; BPH 91; DTXSID0042668; CHEBI:46557; [1-hydroxy-2-(1H-imidazol-1-yl)-1-phosphonoethyl]phosphonic acid; 70HZ18PH24; Zoledronic acid [USAN:INN:BAN]; NCGC00159521-02; (1-hydroxy-2-(1H-imidazol-1-yl)ethylidene)bisphosphonic acid; CGP 42446A; CGP-42446A; Zoledronate hydrate; Phosphonic acid, (1-hydroxy-2-(1H-imidazol-1-yl)ethylidene)bis-; Zoladrona acid mylan; ZOLEDRONIC; Zoledronic acid accord; Zoledronic Acid Actavis; ZOLEDRONIC ACID [INN]; Zoledronic Acid Teva Pharma; Zomera; 1-hydroxy-2-(1H-imidazol-1-yl)ethane-1,1-diyldiphosphonic acid; (1-hydroxy-2-imidazol-1-yl-phosphonoethyl)phosphonic acid monohydrate; Aclasta and Reclast; KS-1132; [1-Hydroxy-2-(1H-imidazol-1-yl)ethylidene]bisphosphonic acid; zoledronic-acid; Aclasta (TN); Zomera (TN); BPH 91 Orazol; CGP 42'446; CGP-42'446; Zoledronic Acid Mylan; [1-HYDROXY-2-(1H-IMIDAZOL-1-YL)-ETHYLIDENE]BISPHOSPHONIC ACID; D0VM2L; Zoledronic acid, Zoledronate; BIDD:PXR0134; SCHEMBL19054; ZOLEDRONIC ACID [MI]; BIDD:GT0292; Zoledronic Acid (Zoledronate); GTPL3177; JMC515594 Compound 55; DTXCID8022668; BDBM12578; CGP42446A; Novartis brand of zoledronic acid; ZOLEDRONIC ACID [WHO-DD]; XRASPMIURGNCCH-UHFFFAOYSA-N; 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic acid; HMS2089O09; BCP22750; CGP-4244; Tox21_111739; C5-H10-N2-O7-P2; HB0674; MFCD00867791; NSC721517; s1314; STL452893; AKOS005145739; AB07564; AC-1092; CS-1829; DB00399; HS-0091; NSC 721517; NCGC00159521-03; NCGC00159521-04; NCGC00159521-05; NCGC00159521-09; NCGC00159521-18; HY-13777; LS-181815; CAS-118072-93-8; FT-0601384; Z0031; D08689; EN300-117269; H11422; S00092; AB01273947-01; AB01273947-02; AB01273947-03; AB01273947_04; A803876; Q218507; SR-05000001436; Q-201946; SR-05000001436-1; Zometa, Zomera, Aclasta and Reclast, Zoledronic Acid; 1-Hydroxy-2-(1-imidazolyl)ethane-1,1-diphosphonic Acid; Z1501485360; (1-Hydroxy-2-(1H-imidazol-1-yl)ethane-1,1-diyl)diphosphonicacid
Click to Show/Hide
|
|||||
| Target(s) | Geranylgeranyl pyrophosphate synthase (GGPS1) | |||||
| Structure |
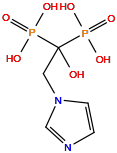
|
|||||
| Formula | C5H10N2O7P2 |
|||||
| Isosmiles | C1=CN(C=N1)CC(O)(P(=O)(O)O)P(=O)(O)O |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14)
|
|||||
| InChIKey |
XRASPMIURGNCCH-UHFFFAOYSA-N
|
|||||
| IUPAC Name |
(1-hydroxy-2-imidazol-1-yl-1-phosphonoethyl)phosphonic acid
|
|||||
| Pharmaceutical Properties | Molecule Weight |
272.09 |
Polar area |
153 |
||
Complexity |
327 |
xlogp Value |
-4.3 |
|||
Heavy Count |
16 |
Rot Bonds |
4 |
|||
Hbond acc |
8 |
Hbond Donor |
5 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
T-47D cells
|
Invasive breast carcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 10000 | nM |
PANC-1 cells
|
Pancreatic ductal adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 10000 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 10000 | nM |
BxPC-3 CDX model cells
|
Pancreatic ductal adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 10000 | nM |
CFPAC-1 cells
|
Pancreatic ductal adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 10000 | nM |
SiHa cells
|
Cervical squamous cell carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 10000 | nM |
Ca-Ski cells
|
Cervical squamous cell carcinoma
|
[2] | |
| Half Maximal Effective Concentration (EC50) | >100000 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | >100000 | nM |
MDA-MB-231 cells (5T4 overexpression)
|
Breast adenocarcinoma
|
[4] | |
| Half Maximal Effective Concentration (EC50) | 10500 | nM |
RPMI-8226 cells
|
Plasma cell myeloma
|
[5] | |
| Half Maximal Effective Concentration (EC50) | 11000 | nM |
RPMI-8226 cells
|
Plasma cell myeloma
|
[6] | |
| Half Maximal Effective Concentration (EC50) | 11000 | nM |
RPMI-8226 cells
|
Plasma cell myeloma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 11700 | nM |
NCI-H460 cells
|
Lung large cell carcinoma
|
[8] | |
| Half Maximal Effective Concentration (EC50) | 13400 | nM |
MIA PaCa-2 cells (MSLN expression)
|
Pancreatic ductal adenocarcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 14300 | nM |
SF268 cells
|
Astrocytoma
|
[8] | |
| Half Maximal Effective Concentration (EC50) | 16100 | nM |
PANC-1 cells
|
Pancreatic ductal adenocarcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | >200000 | nM |
Erythrocyte cells
|
Normal
|
Undisclosed | [10] |
| Half Maximal Effective Concentration (EC50) | 23000 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 23000 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[11] | |
| Half Maximal Inhibitory Concentration (IC50) | 27700 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 34914 | nM |
LoVo cells
|
Colon adenocarcinoma
|
[4] | |
| Half Maximal Effective Concentration (EC50) | 5400 | nM |
T-cells
|
Normal
|
Undisclosed | [12] |
| Half Maximal Inhibitory Concentration (IC50) | 595800 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[11] | |
| Half Maximal Inhibitory Concentration (IC50) | 60000 | nM |
Huh-7 cells
|
Adult hepatocellular carcinoma
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 61582 | nM |
HCT 116 cells
|
Colon carcinoma
|
[4] | |
| Half Maximal Inhibitory Concentration (IC50) | 7800 | nM |
J774 cells
|
Mouse reticulum cell sarcoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 790 | nM |
Human foreskin fibroblasts cells
|
Normal
|
Undisclosed | [15] |
| Half Maximal Effective Concentration (EC50) | 79000 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[3] | |
| Half Maximal Effective Concentration (EC50) | 9400 | nM |
JJN-3 cells
|
Plasma cell myeloma
|
[5] | |
| Half Maximal Effective Concentration (EC50) | 63.7 | uM |
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
[16] |
Each Antibody-drug Conjugate Related to This Payload
References
