Payload Information
General Information of This Payload
| Payload ID | PAY0QNUSP |
|||||
|---|---|---|---|---|---|---|
| Name | Maytansinoid |
|||||
| Synonyms |
Maytansinoid; BP-23647
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
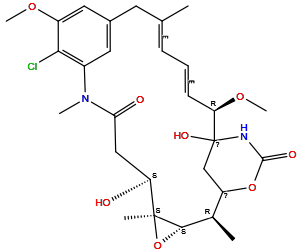
|
|||||
| Formula | C28H37ClN2O8 |
|||||
| Isosmiles | C[C@H]1[C@H]2[C@@](O2)([C@H](CC(=O)N(C3=C(C(=CC(=C3)C/C(=C/C=C/[C@H](C4(CC1OC(=O)N4)O)OC)/C)OC)Cl)C)O)C |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C28H37ClN2O8/c1-15-8-7-9-22(37-6)28(35)14-20(38-26(34)30-28)16(2)25-27(3,39-25)21(32)13-23(33)31(4)18-11-17(10-15)12-19(36-5)24(18)29/h7-9,11-12,16,20-22,25,32,35H,10,13-14H2,1-6H3,(H,30,34)/b9-7+,15-8+/t16-,20?,21+,22-,25+,27+,28?/m1/s1
|
|||||
| InChIKey |
QWPXBEHQFHACTK-ZHGMIJKISA-N
|
|||||
| IUPAC Name |
(2R,3S,5S,6S,16E,18E,20R)-11-chloro-6,21-dihydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaene-8,23-dione
|
|||||
| Pharmaceutical Properties | Molecule Weight |
565.1 |
Polar area |
130 |
||
Complexity |
993 |
xlogp Value |
1.5 |
|||
Heavy Count |
39 |
Rot Bonds |
2 |
|||
Hbond acc |
8 |
Hbond Donor |
3 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.167 | nM |
OVCAR-3 cells
|
Ovarian serous adenocarcinoma
|
[1] |
Each Antibody-drug Conjugate Related to This Payload
References
