Payload Information
General Information of This Payload
| Payload ID | PAY0KXHZI |
|||||
|---|---|---|---|---|---|---|
| Name | Rachelmycin |
|||||
| Synonyms |
Rachelmycin; 69866-21-3; CC-1065; CCRIS 2174; NSC 298223; CC 1065; CHEMBL139109; SCHEMBL9089249; DTXSID40990138; C37H33N7O8; Benzo(1,2-b:4,3-b')dipyrrole-3(2H)-carboxamide, 7-((1,6-dihydro-4-hydroxy-5-methoxy-7-((4,5,8,8a-tetrahydro-7-methyl-4-oxocyclopropa(c)pyrrolo(3,2-e)indol-2(1H)-yl)carbonyl)benzo(1,2-b:4,3-b')dipyrrol-3(2H)-yl)carbonyl)-1,6-dihydro-4-hydroxy-5-methoxy-, (7bR)-; NSC219877; C37-H33-N7-O8; HY-12457; CS-0011401; 5-hydroxy-2-[5-hydroxy-4-methoxy-2-[(1R,12S)-3-methyl-7-oxo-5,10-diazatetracyclo[7.4.0.01,12.02,6]trideca-2(6),3,8-triene-10-carbonyl]-7,8-dihydro-3H-pyrrolo[3,2-e]indole-6-carbonyl]-4-methoxy-7,8-dihydro-3H-pyrrolo[3,2-e]indole-6-carboxamide; Benzo [1,2-b :4,3-b'] dipirrolo-3 (2H)-carboxamida, 7-[[[1,6-dihidro-4-hidroxi-5-metoxi-7-[(7bR, 8sS )-4,5,8,8a-tetrahidro-7-metil-4-oxociclopropa[c] pirrolo [3,2-e] indol-2 (1H)-il] carbonil] benzo [1,2-b: 4,3-b'] dipirrol-3(2H)-il] carbonil]-1,6; Benzo(1,2-b:4,3-b')dipyrrole-3(2H)-carboxamide, 7-((1,6-dihydro-4-hydroxy-5-methoxy-7-((4,5,8,8a-tetrahydro-7-methyl-4-oxocyclopropa(c)pyrrolo(3,2-e)indol-2(1H)-yl)carbonyl)benzo(1,2-b:4,3-b')dipyrrol-3(2H)-yl)carbonyl)-1,6-dihydro-4-hydroxy-5-methox
Click to Show/Hide
|
|||||
| Target(s) | Human Deoxyribonucleic acid (hDNA) | |||||
| Structure |
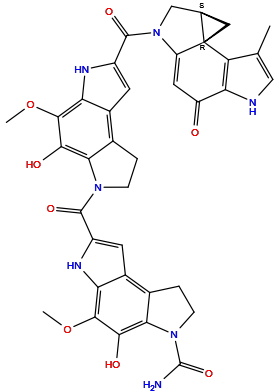
|
|||||
| Formula | C37H33N7O8 |
|||||
| Isosmiles | CC1=CNC2=C1[C@@]34C[C@@H]3CN(C4=CC2=O)C(=O)C5=CC6=C7CCN(C7=C(C(=C6N5)OC)O)C(=O)C8=CC9=C1CCN(C1=C(C(=C9N8)OC)O)C(=O)N |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C37H33N7O8/c1-14-12-39-27-22(45)10-23-37(24(14)27)11-15(37)13-44(23)35(49)21-9-18-16-4-6-42(28(16)30(46)32(51-2)25(18)41-21)34(48)20-8-19-17-5-7-43(36(38)50)29(17)31(47)33(52-3)26(19)40-20/h8-10,12,15,39-41,46-47H,4-7,11,13H2,1-3H3,(H2,38,50)/t15-,37+/m1/s1
|
|||||
| InChIKey |
UOWVMDUEMSNCAV-WYENRQIDSA-N
|
|||||
| IUPAC Name |
5-hydroxy-2-[5-hydroxy-4-methoxy-2-[(1R,12S)-3-methyl-7-oxo-5,10-diazatetracyclo[7.4.0.01,12.02,6]trideca-2(6),3,8-triene-10-carbonyl]-7,8-dihydro-3H-pyrrolo[3,2-e]indole-6-carbonyl]-4-methoxy-7,8-dihydro-3H-pyrrolo[3,2-e]indole-6-carboxamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
703.7 |
Polar area |
210 |
||
Complexity |
1610 |
xlogp Value |
2.4 |
|||
Heavy Count |
52 |
Rot Bonds |
4 |
|||
Hbond acc |
8 |
Hbond Donor |
6 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.00001 | ug/mL |
L1210 cells
|
Lymphoblastic leukemia
|
||
| Half Maximal Inhibitory Concentration (IC50) | 0.026 | ug/mL |
L1210 cells
|
Lymphoblastic leukemia
|
||
| Half Maximal Inhibitory Concentration (IC50) | 0.02 | nM |
L1210 cells
|
Lymphoblastic leukemia
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.07 | nM |
L1210 cells
|
Lymphoblastic leukemia
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 3000 | nM |
L1210 cells
|
Lymphoblastic leukemia
|
[3] |
Each Antibody-drug Conjugate Related to This Payload
References
