Payload Information
General Information of This Payload
| Payload ID | PAY0KEIJX |
|||||
|---|---|---|---|---|---|---|
| Name | TAM470 |
|||||
| Synonyms |
TAM470; SCHEMBL16979627; HY-148128; CS-0612970
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
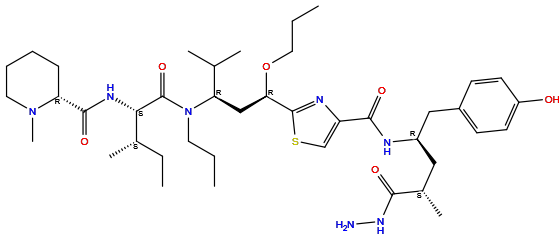
|
|||||
| Formula | C41H67N7O6S |
|||||
| Isosmiles | [H]Oc1c([H])c([H])c(C([H])([H])[C@]([H])(N([H])C(=O)c2nc([C@]([H])(OC([H])([H])C([H])([H])C([H])([H])[H])C([H])([H])[C@@]([H])(N(C(=O)[C@@]([H])(N([H])C(=O)[C@]3([H])N(C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])C3([H])[H])[C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])sc2[H])C([H])([H])[C@@]([H])(C(=O)N([H])N([H])[H])C([H])([H])[H])c([H])c1[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C41H67N7O6S/c1-9-19-48(41(53)36(27(6)11-3)45-39(52)33-14-12-13-20-47(33)8)34(26(4)5)24-35(54-21-10-2)40-44-32(25-55-40)38(51)43-30(22-28(7)37(50)46-42)23-29-15-17-31(49)18-16-29/h15-18,25-28,30,33-36,49H,9-14,19-24,42H2,1-8H3,(H,43,51)(H,45,52)(H,46,50)/t27-,28-,30+,33+,34+,35+,36-/m0/s1
|
|||||
| InChIKey |
WZNRAPVOSYSOAK-WPIAVMABSA-N
|
|||||
| IUPAC Name |
N-[(2R,4S)-5-hydrazinyl-1-(4-hydroxyphenyl)-4-methyl-5-oxopentan-2-yl]-2-[(1R,3R)-4-methyl-3-[[(2S,3S)-3-methyl-2-[[(2R)-1-methylpiperidine-2-carbonyl]amino]pentanoyl]-propylamino]-1-propoxypentyl]-1,3-thiazole-4-carboxamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
786.097 |
Polar area |
179.22 |
||
Complexity |
785.4873539 |
xlogp Value |
5.3421 |
|||
Heavy Count |
55 |
Rot Bonds |
32 |
|||
Hbond acc |
10 |
Hbond Donor |
5 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
OMTX-705 [Phase 1]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 29.40% (Day 50) | High FAP expression (FAP+++) | ||
| Method Description |
Activity of OMTX705 alone or combined with gemcitabine in the Panc 007 PDX model. Five-week-old Foxn1 nu/nu (nude) female mice were implanted subcutaneously with 23 mm 3 tumor pieces of Panc 007,a pancreatic cancer PDX model. OMTX705 was administered intraperitoneally (ip) 10 mg/kg once weekly.
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: Panc 007) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 34.50% (Day 30) | Moderate FAP expression (FAP++) | ||
| Method Description |
Activity of OMTX705 alone or combined with gemcitabine in the TNBC PDX model Breast 014. OMTX705 was given intravenously (iv) 10 mg/kg once weekly for four doses (q7dx4).
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: Breast 014) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 40.70% (Day 30) | Moderate FAP expression (FAP++) | ||
| Method Description |
Activity of OMTX705 alone or combined with gemcitabine in the TNBC PDX model Breast 014. OMTX705 in combination with PTX,OMTX705 intravenous (iv) 10 mg/kg once weekly for four doses (q7dx4). PTX intravenous (iv) 7.5 mg/kg once weekly for three doses (q7dx3) starting from week two.
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: Breast 014) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 49.30% (Day 37) | Moderate FAP expression (FAP++) | ||
| Method Description |
Activity of OMTX705 alone or combined with gemcitabine in the Lung 024 PDX model. Five-week-old Foxn1 nu/nu (nude) female mice were implanted subcutaneously with 23 mm 3 tumor pieces of Panc 007,a pancreatic cancer PDX model. OMTX705 was administered intraperitoneally (ip) at 10 mg/kg,twice in the first week.
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: Lung 024) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 57.50% (Day 37) | Moderate FAP expression (FAP++) | ||
| Method Description |
Activity of OMTX705 alone or combined with gemcitabine in the Lung 024 PDX model. Lung adenocarcinoma Lung 024 PDX models treated with OMTX705 alone or combined with paclitaxel (PTX). OMTX705 and PTX were combined at a dose of 7.5 mg/kg of PTX administered intravenously once a week starting in the second week,and OMTX705 at a dose of 10 mg/kg administered by injection twice in the first week.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: Lung 024) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.70% (Day 50) | High FAP expression (FAP+++) | ||
| Method Description |
Activity of OMTX705 alone or combined with gemcitabine in the Panc 007 PDX model. Five-week-old Foxn1 nu/nu (nude) female mice were implanted subcutaneously with 23 mm 3 tumor pieces of Panc 007,a pancreatic cancer PDX model. OMTX705 was administered intraperitoneally (ip) 20 mg/kg once weekly.
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: Panc 007) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.60% (Day 37) | Moderate FAP expression (FAP++) | ||
| Method Description |
Activity of OMTX705 alone or combined with gemcitabine in the Lung 024 PDX model. Lung adenocarcinoma Lung 024 PDX models treated with OMTX705 alone or combined with paclitaxel (PTX). OMTX705 was administered intraperitoneally (ip) at 30 mg/kg,once per week.
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: Lung 024) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 74.00% (Day 33) | High FAP expression (FAP+++) | ||
| Method Description |
OMTX705 exhibits potent effect in immunodeficient PDX models of different solid tumors. Five-week-old Foxn1 nu/nu (nude) female mice were implanted subcutaneously with 23 mm 3 tumor pieces of Panc 007,a pancreatic cancer PDX model. OMTX705 was given intravenously (iv) 20 mg/kg once weekly for four doses (q7dx4).
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: Panc 007) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.40% (Day 30) | Moderate FAP expression (FAP++) | ||
| Method Description |
Activity of OMTX705 alone or combined with gemcitabine in the TNBC PDX model Breast 014. OMTX705 was given intravenously (iv) 30 mg/kg once weekly for four doses (q7dx4).
|
||||
| In Vivo Model | Triple-negative breast cancer PDX model (PDX: Breast 014) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.90% (Day 33) | High FAP expression (FAP+++) | ||
| Method Description |
OMTX705 exhibits potent effect in immunodeficient PDX models of different solid tumors. Five-week-old Foxn1 nu/nu (nude) female mice were implanted subcutaneously with 23 mm 3 tumor pieces of Panc 007,a pancreatic cancer PDX model. OMTX705 was given intravenously (iv) 30 mg/kg once weekly for four doses (q7dx4).
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: Panc 007) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 85.90% (Day 33) | High FAP expression (FAP+++) | ||
| Method Description |
OMTX705 exhibits potent effect in immunodeficient PDX models of different solid tumors. Five-week-old Foxn1 nu/nu (nude) female mice were implanted subcutaneously with 23 mm 3 tumor pieces of Panc 007,a pancreatic cancer PDX model. OMTX705 was given intravenously (iv) 40 mg/kg once weekly for four doses (q7dx4).
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: Panc 007) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.50% (Day 50) | High FAP expression (FAP+++) | ||
| Method Description |
Activity of OMTX705 alone or combined with gemcitabine in the Panc 007 PDX model. Five-week-old Foxn1 nu/nu (nude) female mice were implanted subcutaneously with 23 mm 3 tumor pieces of Panc 007,a pancreatic cancer PDX model. OMTX705 was administered intraperitoneally (ip) 10 mg/kg once weekly,whereas gemcitabine was administered intraperitoneally 50 mg/kgweekly starting in the second week.
Click to Show/Hide
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: Panc 007) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.10% (Day 33) | High FAP expression (FAP+++) | ||
| Method Description |
OMTX705 exhibits potent effect in immunodeficient PDX models of different solid tumors. Five-week-old Foxn1 nu/nu (nude) female mice were implanted subcutaneously with 23 mm 3 tumor pieces of Panc 007,a pancreatic cancer PDX model. OMTX705 was given intravenously (iv) 50 mg/kg once weekly for four doses (q7dx4).
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: Panc 007) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.10% (Day 33) | High FAP expression (FAP+++) | ||
| Method Description |
OMTX705 exhibits potent effect in immunodeficient PDX models of different solid tumors. Five-week-old Foxn1 nu/nu (nude) female mice were implanted subcutaneously with 23 mm 3 tumor pieces of Panc 007,a pancreatic cancer PDX model. OMTX705 was given intravenously (iv) 60 mg/kg once weekly for four doses (q7dx4).
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: Panc 007) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
230.00 pM
|
High FAP expression (FAP+++) | ||
| Method Description |
Cell viability in CAF07, HT1080-FAP, and HT1080-WT cells was analyzed via crystal violet staining after exposure to the indicated antibodies or free drug. Cells were treated for 120 hours with a serial dilution of antibodies (starting at 400 nmol/L) or free drugs (starting at 60 mol/L).
|
||||
| In Vitro Model | Fibrosarcoma | HT-1080 cells (FAP expression) | CVCL_0317 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
400.00 nM
|
Moderate FAP expression (FAP++) | ||
| Method Description |
Cell viability in CAF07, HT1080-FAP, and HT1080-WT cells was analyzed via crystal violet staining after exposure to the indicated antibodies or free drug. Cells were treated for 120 hours with a serial dilution of antibodies (starting at 400 nmol/L) or free drugs (starting at 60 mol/L).
|
||||
| In Vitro Model | Normal | CAF cells | CVCL_R883 | ||
References
