Payload Information
General Information of This Payload
| Payload ID | PAY0JYSRJ |
|||||
|---|---|---|---|---|---|---|
| Name | Auristatin M |
|||||
| Synonyms |
Auristatin M
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
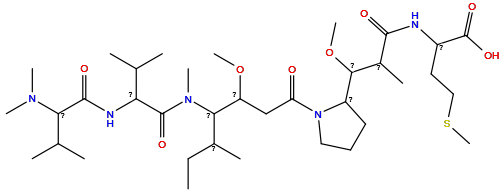
|
|||||
| Formula | C36H67N5O8S |
|||||
| Isosmiles | CCC(C)C(C(CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(CCSC)C(=O)O)OC)N(C)C(=O)C(NC(=O)C(C(C)C)N(C)C)C(C)C |
|||||
| InChI |
InChI=1S/C36H67N5O8S/c1-14-23(6)31(40(10)35(45)29(21(2)3)38-34(44)30(22(4)5)39(8)9)27(48-11)20-28(42)41-18-15-16-26(41)32(49-12)24(7)33(43)37-25(36(46)47)17-19-50-13/h21-27,29-32H,14-20H2,1-13H3,(H,37,43)(H,38,44)(H,46,47)
|
|||||
| InChIKey |
STYIZAGDTRHYON-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
730.026 |
Polar area |
157.82 |
||
Complexity |
50 |
xlogp Value |
2.9562 |
|||
Heavy Count |
50 |
Rot Bonds |
22 |
|||
Hbond acc |
9 |
Hbond Donor |
3 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
1F6-Asn- (D)Lys-AM [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
93.91% (Day 30)
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
1 mg mAb component/kg in vivo therapeutic efficacy of the conjugates in nude mice(5 animals/group).
|
||||
| In Vivo Model | 786-O human RCC cell line xenograft model | ||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.00 ng/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
IC50 values are the mean and SD of 21 independent experimental titrations, each performed at least in duplicate.
|
||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
20.00 ng/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
IC50 values are the mean and SD of 21 independent experimental titrations, each performed at least in duplicate.
|
||||
| In Vitro Model | Clear cell renal cell carcinoma | Caki-1 cells | CVCL_0234 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
28.00 ng/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
IC50 values are the mean and SD of 21 independent experimental titrations, each performed at least in duplicate.
|
||||
| In Vitro Model | Hodgkin lymphoma | L-428 cells | CVCL_1361 | ||
1F6-Met- (D)Lys-AM [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
39.00 ng/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
IC50 values are the mean and SD of 21 independent experimental titrations, each performed at least in duplicate.
|
||||
| In Vitro Model | Clear cell renal cell carcinoma | Caki-1 cells | CVCL_0234 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
56.00 ng/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
IC50 values are the mean and SD of 21 independent experimental titrations, each performed at least in duplicate.
|
||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1000.00 ng/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
IC50 values are the mean and SD of 21 independent experimental titrations, each performed at least in duplicate.
|
||||
| In Vitro Model | Hodgkin lymphoma | L-428 cells | CVCL_1361 | ||
