Payload Information
General Information of This Payload
| Payload ID | PAY0IJSCK |
|||||
|---|---|---|---|---|---|---|
| Name | PBD dimer |
|||||
| Synonyms |
PBD dimer; SGD-1882; 1222490-34-7; IRE1I9FE08; (6aS)-3-[3-[[(6aS)-2-methoxy-8-(4-methoxyphenyl)-11-oxo-6a,7-dihydropyrrolo[2,1-c][1,4]benzodiazepin-3-yl]oxy]propoxy]-8-(4-aminophenyl)-2-methoxy-6a,7-dihydropyrrolo[2,1-c][1,4]benzodiazepin-11-one; J3.194.362K; 5H-Pyrrolo(2,1-C)(1,4)benzodiazepin-5-one, 2-(4-aminophenyl)-8-(3-(((11aS)-5,11a-dihydro-7-methoxy-2-(4-methoxyphenyl)-5-oxo-1H-pyrrolo(2,1-C)(1,4)benzodiazepin-8-yl)oxy)propoxy)-1,11a-dihydro-7-methoxy-, (11aS)-; 5H-Pyrrolo[2,1-c][1,4]benzodiazepin-5-one, 2-(4-aminophenyl)-8-[3-[[(11aS)-5,11a-dihydro-7-methoxy-2-(4-methoxyphenyl)-5-oxo-1H-pyrrolo[2,1-c][1,4]benzodiazepin-8-yl]oxy]propoxy]-1,11a-dihydro-7-methoxy-, (11aS)-; 8-(3-((2-(4-Aminophenyl)-7-methoxy-5-oxo-1,11abeta-dihydro-5H-pyrrolo(2,1-C)(1,4)benzodiazepine-8-yl)oxy)propoxy)-7-methoxy-2-(4-methoxyphenyl)-1,11abeta-dihydro-5H-pyrrolo(2,1-C)(1,4)benzodiazepine-5-one; 8-[3-[[2-(4-Aminophenyl)-7-methoxy-5-oxo-1,11abeta-dihydro-5H-pyrrolo[2,1-c][1,4]benzodiazepine-8-yl]oxy]propoxy]-7-methoxy-2-(4-methoxyphenyl)-1,11abeta-dihydro-5H-pyrrolo[2,1-c][1,4]benzodiazepine-5-one; UNII-IRE1I9FE08; SCHEMBL2291136; OMRPLUKQNWNZAV-CONSDPRKSA-N; EX-A6227; SGD 1882; CS-7766; BP-29355; MS-31248; PD126348; HY-101127; Q27280866; (S)-2-(4-aminophenyl)-7-methoxy-8-(3-((S)-7-methoxy-2-(4-methoxyphenyl)-5-oxo-5,11a-dihydro-1H-pyrrolo[2,1-c][1,4]benzodiazepine-8-yloxy)propoxy)-1H-pyrrolo[2,1-c][1,4]benzodiazepine-5(11aH)-one; 8-(3-((2-(4-AMINOPHENYL)-7-METHOXY-5-OXO-1,11A.BETA.-DIHYDRO-5H-PYRROLO(2,1-C)(1,4)BENZODIAZEPINE-8-YL)OXY)PROPOXY)-7-METHOXY-2-(4-METHOXYPHENYL)-1,11A.BETA.-DIHYDRO-5H-PYRROLO(2,1-C)(1,4)BENZODIAZEPINE-5-ONE
Click to Show/Hide
|
|||||
| Target(s) | Human Deoxyribonucleic acid (hDNA) | |||||
| Structure |
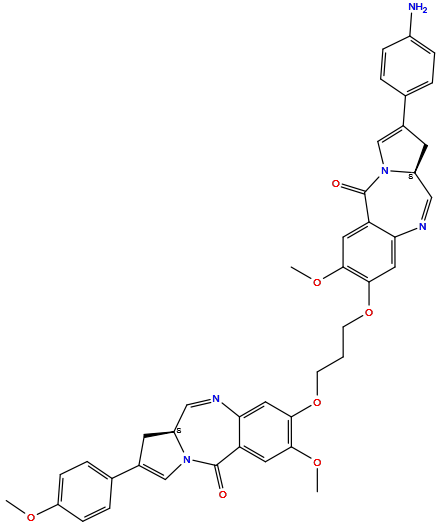
|
|||||
| Formula | C42H39N5O7 |
|||||
| Isosmiles | [H]C1=Nc2c([H])c(OC([H])([H])C([H])([H])C([H])([H])Oc3c([H])c4c(c([H])c3OC([H])([H])[H])C(=O)N3C([H])=C(c5c([H])c([H])c(N([H])[H])c([H])c5[H])C([H])([H])[C@@]3([H])C([H])=N4)c(OC([H])([H])[H])c([H])c2C(=O)N2C([H])=C(c3c([H])c([H])c(OC([H])([H])[H])c([H])c3[H])C([H])([H])[C@@]12[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C42H39N5O7/c1-50-32-11-7-26(8-12-32)28-16-31-22-45-36-20-40(38(52-3)18-34(36)42(49)47(31)24-28)54-14-4-13-53-39-19-35-33(17-37(39)51-2)41(48)46-23-27(15-30(46)21-44-35)25-5-9-29(43)10-6-25/h5-12,17-24,30-31H,4,13-16,43H2,1-3H3/t30-,31-/m0/s1
|
|||||
| InChIKey |
OMRPLUKQNWNZAV-CONSDPRKSA-N
|
|||||
| IUPAC Name |
(6aS)-3-[3-[[(6aS)-2-methoxy-8-(4-methoxyphenyl)-11-oxo-6a,7-dihydropyrrolo[2,1-c][1,4]benzodiazepin-3-yl]oxy]propoxy]-8-(4-aminophenyl)-2-methoxy-6a,7-dihydropyrrolo[2,1-c][1,4]benzodiazepin-11-one
|
|||||
| Pharmaceutical Properties | Molecule Weight |
725.802 |
Polar area |
137.51 |
||
Complexity |
725.2849486 |
xlogp Value |
7.0855 |
|||
Heavy Count |
54 |
Rot Bonds |
15 |
|||
Hbond acc |
10 |
Hbond Donor |
1 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | <0.02 | mg/L |
MCF-7 cells
|
Invasive breast carcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | <0.02 | mg/L |
A2780 cells
|
Ovarian endometrioid adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.08-0.16 | mg/L |
WI-38 cells
|
Normal
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 34.67 | nM |
Daudi cells
|
Burkitt lymphoma
|
[2] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
SGN-CD70A [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
20.00%
|
Positive CD70 expression (CD70+++/++) | ||
| Patients Enrolled |
CD70-positive MCL or DLBCL including FL3b (expression in at least 50% of the sample)
|
||||
| Administration Dosage |
8 mg/kg (up to a maximum of 200 mg) intravenously once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02216890 | Phase Status | Phase 1 | ||
| Clinical Description |
Safety study of SGN-CD70A in cancer patients.
|
||||
| Primary Endpoint |
Objective response rate=20.00% (95% CI 5.70-43.70).
|
||||
| Other Endpoint |
Median progression free survival=1.90 months.
|
||||
ABBV-322 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 34.60% (Day 28) | Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
For the PDX 14R091 and PDX MPM36 studies, mice received ABBV-322 (0.03 mg/kg) or control ADC (0.03 mg/kg) every 4 days, for a total of 12 treatments.
|
||||
| In Vivo Model | Malignant Mesothelioma PDX model (PDX: MPM36) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 65.80% (Day 60) | Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
For the PDX 14R091 and PDX MPM36 studies, mice received ABBV-322 (0.03 mg/kg) or control ADC (0.03 mg/kg) every 4 days, for a total of 12 treatments.
|
||||
| In Vivo Model | Malignant Mesothelioma PDX model (PDX: 14R091) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 ug/mL - 35.00 ug/mL
|
Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
Cells lines were plated at 1,000-3,000 cells per well in complete growth medium containing 10% FCS in 96-well plates and allowed to adhere overnight.
|
||||
| In Vitro Model | Pleural biphasic mesothelioma | MSTO-211H cells | CVCL_1430 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 ug/mL - 35.00 ug/mL
|
Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
Cells lines were plated at 1,000-3,000 cells per well in complete growth medium containing 10% FCS in 96-well plates and allowed to adhere overnight.
|
||||
| In Vitro Model | Pleural mesothelioma | NCI-H28 cells | CVCL_1555 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 ug/mL - 35.00 ug/mL
|
Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
Cells lines were plated at 1,000-3,000 cells per well in complete growth medium containing 10% FCS in 96-well plates and allowed to adhere overnight.
|
||||
| In Vitro Model | Pleural mesothelioma | NCI-H2052 cells | CVCL_1518 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 ug/mL - 35.00 ug/mL
|
Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
Cells lines were plated at 1,000-3,000 cells per well in complete growth medium containing 10% FCS in 96-well plates and allowed to adhere overnight.
|
||||
| In Vitro Model | Pleural mesothelioma | NCI-H2052 cells | CVCL_1518 | ||
ABT-700 (S238C)-PBD [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
70.68% (Day 36)
|
Low MET expression (MET+; IHC 1+) | ||
| Method Description |
Tumor fragments of 3 to 5 mm at passage 3 were implanted subcutaneously in the right rear flank of NSG mice with a trochar. ABT-700 PBD was administered 0.3 mg/kg every seven days for a total of six doses.
|
||||
| In Vivo Model | Non-small cell lung cancer PDX model (PDX: CTG-0363) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
75.79% (Day 22)
|
High MET expression (MET+++; IHC 3+) | ||
| Method Description |
Tumor fragments of 3 to 5 mm at passage 3 were implanted subcutaneously in the right rear flank of NSG mice with a trochar. ABT-700 PBD was administered 0.3 mg/kg every seven days for a total of six doses.
|
||||
| In Vivo Model | Non-small cell lung cancer PDX model (PDX: CTG-0170) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
88.94% (Day 21)
|
Moderate MET expression (MET++; IHC 2+) | ||
| Method Description |
Tumor fragments of 3 to 5 mm at passage 3 were implanted subcutaneously in the right rear flank of NSG mice with a trochar. ABT-700 PBD was administered 0.3 mg/kg every seven days for a total of six doses.
|
||||
| In Vivo Model | Non-small cell lung cancer PDX model (PDX: CTG-0159) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
92.94% (Day 32)
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Mice were randomized when the tumors reached -200 mm and dosed on day 0 with isotype or ADC at 0.3 mg/kg intraperitoneally.
|
||||
| In Vivo Model | SW48 CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | SK-CO-1 cells | CVCL_0626 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | LS174T cells | CVCL_1384 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 1.00 pM | Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Astrocytoma | U-138MG cells | CVCL_0020 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 1.00 pM | Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Glioblastoma | M059J cells | CVCL_0400 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 1.00 pM | Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Glioblastoma | M059K cells | CVCL_0401 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 1.00 pM | Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Anaplastic astrocytoma | DBTRG-05MG cells | CVCL_1169 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.90 pM
|
Low MET expression (MET+) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | HCC15 cells | CVCL_2057 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | DLD-1 cells | CVCL_0248 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | HT-29 cells | CVCL_0320 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon carcinoma | HCT 116 cells | CVCL_0291 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW620 cells | CVCL_0547 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Gliosarcoma | SF264 cells | Homo sapiens | ||
| Experiment 14 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW403 cells | CVCL_0545 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Astrocytoma | SNB-19 cells | CVCL_0535 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
7.00 pM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | COLO 201 cells | CVCL_1987 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 7.00 pM | Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Astrocytoma | U-251MG cells | CVCL_0021 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
High MET expression (MET+++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung papillary adenocarcinoma | NCI-H441 cells | CVCL_1561 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon carcinoma | RKO cells | CVCL_0504 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Rectal adenocarcinoma | SW1463 cells | CVCL_1718 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | WiDr cells | CVCL_2760 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
High MET expression (MET+++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Gastric adenocarcinoma | Hs 746.T cells | CVCL_0333 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Low MET expression (MET+) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Glioblastoma | M059J cells | CVCL_0400 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Low MET expression (MET+) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Pancreatic carcinoma | KP-4 cells | CVCL_1338 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung papillary adenocarcinoma | NCI-H820 cells | CVCL_1592 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | SW900 cells | CVCL_1731 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | COLO 205 cells | CVCL_0218 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | COLO 320DM cells | CVCL_0219 | ||
| Experiment 29 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW1116 cells | CVCL_0544 | ||
| Experiment 30 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Pleural epithelioid mesothelioma | NCI-H226 cells | CVCL_1544 | ||
| Experiment 31 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Cecum adenocarcinoma | LS1034 cells | CVCL_1382 | ||
| Experiment 32 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | COLO 320HSR cells | CVCL_0220 | ||
| Experiment 33 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Gliosarcoma | SF539 cells | CVCL_1691 | ||
| Experiment 34 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1573 cells | CVCL_1478 | ||
| Experiment 35 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | LoVo cells | CVCL_0399 | ||
| Experiment 36 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
High MET expression (MET+++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | EBC-1 cells | CVCL_2891 | ||
| Experiment 37 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Low MET expression (MET+) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 38 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Low MET expression (MET+) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 39 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | T84 cells | CVCL_0555 | ||
| Experiment 40 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.17 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | SK-MES-1 cells | CVCL_0630 | ||
| Experiment 41 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20 nM
|
Low MET expression (MET+) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Astrocytoma | U-118MG cells | CVCL_0633 | ||
| Experiment 42 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.21 nM
|
Low MET expression (MET+) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Glioblastoma | U-87MG cells | CVCL_0022 | ||
| Experiment 43 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.40 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1650 cells | CVCL_1483 | ||
| Experiment 44 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.70 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H1703 cells | CVCL_1490 | ||
| Experiment 45 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.97 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Anaplastic astrocytoma | CHLA-03-AA cells | CVCL_U616 | ||
| Experiment 46 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.45 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Primitive neuroectodermal tumor | PFSK-1 cells | CVCL_1642 | ||
| Experiment 47 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
26.00 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW480 cells | CVCL_0546 | ||
| Experiment 48 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
28.20 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Glioblastoma | SNB-75 cells | CVCL_1706 | ||
| Experiment 49 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 67.00 nM | Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | Caco-2 cells | CVCL_0025 | ||
| Experiment 50 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 67.00 nM | Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Colon adenocarcinoma | HCT 15 cells | CVCL_0292 | ||
| Experiment 51 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 67.00 nM | Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Glioblastoma | A-172 cells | CVCL_0131 | ||
| Experiment 52 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
141 nM
|
Positive MET expression (MET +++/++) | ||
| Method Description |
Cancer cell lines were incubated with compounds for 72 h. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Glioblastoma | T98G cells | CVCL_0556 | ||
D3-GPC2-PBD [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 86.80% (Day 2) | High GPC2 expression (GPC2+++) | ||
| Method Description |
Tumors were typically implanted into the flanks of female 5-9 week-old C.B-17 scid mice. Each mouse was then given a single dose of their respective ADC treatments in PBS or vehicle (Day 0) via intraperitoneal (IP) injection. In some efficacy studies, one cohort of mice received 3 subsequent ADC 1 mg/kg IP injections over the following 2 weeks (1 mg/kg x 4 cohort).
Click to Show/Hide
|
||||
| In Vivo Model | COG-N-421x PDX model | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 pM
|
High GPC2 expression (GPC2+++) | ||
| Method Description |
Cells were incubated with increasing concentrations in tested compounds for 96 h and cell viability was determined by MTS assay.
|
||||
| In Vitro Model | Neuroblastoma | SK-N-AS cells | CVCL_1700 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
26.60 pM
|
Positive GPC2 expression (GPC2 +++/++) | ||
| Method Description |
Cell lines were plated in 96-well plates (typically between 1,000 and 5,000 cells/well) and treated with serial dilutions of each ADC payload, the D3-GPC2-PBD ADC, or vehicle the following day. After four additional days, cell viability was determined using a CellTiter-Glo Assay.
|
||||
| In Vitro Model | Neuroblastoma | NB-SD cells | CVCL_LF68 | ||
HuM25-S239C-PBD-E2 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
60.13% (Day 13)
|
High LRRC15 expression (LRRC15+++; IHC 3+) | ||
| Method Description |
EBC-1 squamous NSCLC cells (5 million) were implanted subcutaneously into SCID mice, and micewere randomized when the tumors reached 175 mm and dosed with ADC or isotype antibody at 0.6 mg/kg intraperitoneally on day 0.
|
||||
| In Vivo Model | EBC-1 CDX model | ||||
| In Vitro Model | Lung squamous cell carcinoma | EBC-1 cells | CVCL_2891 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
73.58% (Day 17)
|
High LRRC15 expression (LRRC15+++; IHC 3+) | ||
| Method Description |
NCI-H1650 NSCLC cancer cells (5 million) were implanted subcutaneously into SCID/Beigemice. and mice were randomized when the tumors reached -200 mm and dosed on day 0 with isotype orADC at 0.1 mg/kg intraperitoneally.
|
||||
| In Vivo Model | NCI-H1650 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1650 cells | CVCL_1483 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
92.33% (Day 17)
|
High LRRC15 expression (LRRC15+++; IHC 3+) | ||
| Method Description |
NCI-H1650 NSCLC cancer cells (5 million) were implanted subcutaneously into SCID/Beigemice. and mice were randomized when the tumors reached -200 mm and dosed on day 0 with isotype orADC at 0.3 mg/kg intraperitoneally.
|
||||
| In Vivo Model | NCI-H1650 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1650 cells | CVCL_1483 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
94.51% (Day 17)
|
High LRRC15 expression (LRRC15+++; IHC 3+) | ||
| Method Description |
NCI-H1650 NSCLC cancer cells (5 million) were implanted subcutaneously into SCID/Beigemice. and mice were randomized when the tumors reached -200 mm and dosed on day 0 with isotype orADC at 6 mg/kg intraperitoneally.
|
||||
| In Vivo Model | NCI-H1650 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1650 cells | CVCL_1483 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00 pM
|
Positive LRRC15 expression (LRRC15 +++/++) | ||
| Method Description |
In vitro cell killing in LRRC15 transfected 3T12 cells by isotype-S239C-PBD-E2 or huM25-S239CPBD-E2. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Squamous non-small cell lung cancer | BALB/3T12-3 cells (huLRRC15 transfection) | CVCL_0637 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 nM-100.00 Nm
|
Positive LRRC15 expression (LRRC15 +++/++) | ||
| Method Description |
In vitro cell killing in A549 cells that have undergone epithelial tomesenchymal transition (EMT) in the presence of 10 ng/mL TGFB by isotype-S239C-PBD-E2huM25-S239C-PBD-E2, or huM25-S239C antibody.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 nM-100.00 Nm
|
Positive LRRC15 expression (LRRC15 +++/++) | ||
| Method Description |
In vitro cell killing in A549 lung cancer cells in the presence of 10 ng/mL TGFB by isotype-S239C-PBD-E2huM25-S239C-PBD-E2, or huM25-S239C antibody.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
Anti-ApoD PBD [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% | Positive APOD expression (APOD +++/++) | ||
| Method Description |
Male Bl6 mice, at 9 weeks old (young) or 80 weeks old (old), were intravenously treated with the vehicle alone or with anti-ApoD antibody and PBD-conjugated IgG with a cleavable linker, each at a concentration of 0.3 mg/kg and 3 mg/kg in a single dose.
|
||||
| In Vivo Model | NHDF CDX model | ||||
| In Vitro Model | Normal | NHDF cells | Homo sapiens | ||
HuAD208.4.1-PBD-DAR2 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
91.00% (Day 18)
|
High LRRC15 expression (LRRC15+++; IHC 3+) | ||
| Method Description |
EBC-1 squamous NSCLC cells (5 million) were implanted subcutaneously into SCID mice, and mice were randomized when the tumors reached 225 mm and dosed with ADC at 0.6 mg/kg Q7Dx2 (one dose given every 7 days for a total of 2 doses) or isotype antibody at 6 mg/kg intraperitoneally starting on day 0.
|
||||
| In Vivo Model | EBC-1 CDX model | ||||
| In Vitro Model | Lung squamous cell carcinoma | EBC-1 cells | CVCL_2891 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
95.80% (Day 26)
|
High LRRC15 expression (LRRC15+++; IHC 3+) | ||
| Method Description |
NCI-H1650 adeno NSCLCcells (5 million) were implanted subcutaneously into SCID/Beige mice, and mice were randomized whenthe tumors reached 225 mm and dosed with ADC at 0.6 mg/kg or isotype antibody at 12 mg/kg intraperitoneally once on day 0.
|
||||
| In Vivo Model | NCI-H1650 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1650 cells | CVCL_1483 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 nM
|
Positive LRRC15 expression (LRRC15 +++/++) | ||
| Method Description |
In vitro cell killing in murine Balb/c BM-MSC (Cyagen) mesenchymal stem cells in thepresence of 10 ng/mL TGF by isotype-PBD-DAR2 or huAD208.4.1-PBD-DAR2. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Normal | Mouse bone marrow-derived mesenchymal stem (BM-MSC) cells | Mus musculus | ||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 nM-100.00 Nm
|
Positive LRRC15 expression (LRRC15 +++/++) | ||
| Method Description |
In vitro cell killing in human BM-MSC (Lonza) mesenchymalstem cells in the presence of 10 ng/mL TGFB by isotype-PBD-DAR2 or huAD208.4.1-PBD-DAR2. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Normal | Human bone marrow-derived mesenchymal stem (BM-MSC) cells | Homo sapiens | ||
References
